

PAYERS COVER RINVOQ

PREFERRED COMMERCIAL AND MEDICARE PART D COVERAGE 2,*,†
National Commercial and Medicare Part D formulary coverage under the pharmacy benefit as of April 2024 2
Preferred coverage also could mean a STANDARD PRIOR AUTHORIZATION (PA) and APPEALS PROCESS, potential for one-time PA/appeal approval.
*RINVOQ is on a preferred tier or otherwise has preferred status on the plan’s formulary.
† Coverage requirements and benefit designs vary by payer and may change over time.
JAKi=Janus kinase inhibitor; PI=prescribing information; RA=rheumatoid arthritis; TNFi=tumor necrosis factor inhibitor
Preferred coverage also could mean a
STANDARD PRIOR AUTHORIZATION (PA)
and APPEALS PROCESS, potential for one-time PA/appeal approval.

Our Nurse Ambassadors are the heart of RINVOQ Complete and HUMIRA Complete
Nurse Ambassadors provide 1:1 support to help patients start and stay on track with their prescribed treatment plan, including:

Insurance support when needed

Help with access &
treatment affordability
Complete can help your commercial patients save:
Have questions or need support over the phone?
Call 1-877-COMPLETE (1‑80 0‑274‑6867)
*Nurse Ambassadors are provided by AbbVie and do not provide medical advice or work under the direction of the prescribing healthcare professional (HCP). They are trained to direct patients to speak with their HCP about any treatment‑related questions, including further referrals.
† Eligibility: Available to patients with commercial insurance coverage for RINVOQ® (upadacitinib), or HUMIRA® (adalimumab) who meet eligibility criteria. This co-pay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs) or where prohibited by law. Offer subject to change or termination without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. For full Terms and Conditions, visit RINVOQSavingsCard.com for RINVOQ® patients, and HUMIRASavingsCard.com for HUMIRA® patients, or call 1.800.2RINVOQ or 1.800.4HUMIRA for additional information To learn about AbbVie’s privacy practices and your privacy choices, visit https://abbv.ie/corpprivacy
‡ Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for RINVOQ® (upadacitinib) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for RINVOQ® (upadacitinib) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.
To enroll a patient in RINVOQ Complete:

Pull your patient’s patient demographic sheet from your Electronic Health Record

Download the enrollment & prescription form for your specialty

Fill out the form with your patient

Fax the enrollment & prescription form and the patient demographic sheet to 1-678-727-0690

Inform your patient that they have been enrolled
* Eligibility criteria: Available to patients aged 63 or younger with commercial insurance coverage. Patients must have a valid prescription for RINVOQ® (upadacitinib) for an FDA approved indication and a denial of insurance coverage based on a prior authorization request on file along with a confirmation of appeal. Continued eligibility for the program requires the submission of an appeal of the coverage denial every 180 days. Program provides for RINVOQ® (upadacitinib) at no charge to patients for up to two years or until they receive insurance coverage approval, whichever occurs earlier, and is not contingent on purchase requirements of any kind. Program is not available to patients whose medications are reimbursed in whole or in part by Medicare, Medicaid, TRICARE, or any other federal or state program. Offer subject to change or discontinuance without notice. This is not health insurance and program does not guarantee insurance coverage. No claims for payment may be submitted to any third party for product dispensed by program. Limitations may apply.

The Complete App is designed to help patients stay on track with their prescribed RINVOQ or HUMIRA treatment by helping them:



Streamline the Rx process
CompletePro.com enables seamless enrollment into RINVOQ Complete and HUMIRA Complete, and helps streamline the prescription process for your patients.
With CompletePro.com, you can:
Download RINVOQ
resources to support
your
patients and your practice.
Review the well-studied safety profile of RINVOQ,
including both short- and long-term analyses

RINVOQ is indicated for the treatment of:
Limitations of Use: RINVOQ is not recommended for use in combination with other Janus kinase (JAK) inhibitors, biologic disease-modifying antirheumatic drugs (bDMARDs), or with potent immunosuppressants such as azathioprine and cyclosporine.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or other immunosuppressants.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biological therapies for ulcerative colitis or Crohn’s disease, or with potent immunosuppressants such as azathioprine and cyclosporine.
Patients treated with RINVOQ* are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants, such as methotrexate or corticosteroids. If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
Carefully consider the risks and benefits of treatment with RINVOQ prior to initiating therapy in patients with chronic or recurrent infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
In a large, randomized, postmarketing safety study comparing another Janus kinase (JAK) inhibitor with tumor necrosis factor (TNF) blockers in rheumatoid arthritis (RA) patients ≥50 years old with at least one cardiovascular (CV) risk factor, a higher rate of all-cause mortality, including sudden CV death, was observed with the JAK inhibitor. Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Lymphoma and other malignancies have been observed in patients treated with RINVOQ.
In a large, randomized, postmarketing safety study comparing another JAK inhibitor with TNF blockers in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]), lymphomas, and lung cancer (in current or past smokers) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk.
With RINVOQ, consider the benefits and risks for the individual patient prior to initiating or continuing therapy, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers. NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer. Advise patients to limit sunlight exposure by wearing protective clothing and using sunscreen.
In a large, randomized, postmarketing study comparing another JAK inhibitor with TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of MACE (defined as cardiovascular death, myocardial infarction, and stroke) was observed with the JAK inhibitor. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other CV risk factors. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing study comparing another JAK inhibitor to TNF blockers in RA patients ≥50 years old with at least one CV risk factor, a higher rate of thrombosis was observed with the JAK inhibitor. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients. Serious hypersensitivity reactions, such as anaphylaxis and angioedema, were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
Gastrointestinal (GI) perforations have been reported in clinical trials with RINVOQ. Monitor RINVOQ-treated patients who may be at risk for GI perforation (e.g., patients with a history of diverticulitis and patients taking NSAIDs or corticosteroids). Promptly evaluate patients presenting with new onset abdominal pain for early identification of GI perforation.
Neutropenia
Lymphopenia
Treatment with RINVOQ was associated with increases in lipid parameters, including total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol. Manage patients according to clinical guidelines for the management of hyperlipidemia. Evaluate patients 12 weeks after initiation of treatment and thereafter according to the clinical guidelines for hyperlipidemia.
Liver enzyme elevations
Treatment with RINVOQ was associated with increased incidence of liver enzyme elevation compared to placebo. Evaluate at baseline and thereafter according to routine patient management. Prompt investigation of the cause of liver enzyme elevation is recommended to identify potential cases of drug-induced liver injury. If increases in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) are observed during routine patient management and drug-induced liver injury is suspected, RINVOQ should be interrupted until this diagnosis is excluded.
Based on findings in animal studies, RINVOQ may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with RINVOQ and for 4 weeks after the final dose. Verify pregnancy status of females of reproductive potential prior to starting treatment with RINVOQ.
Avoid use of live vaccines during, or immediately prior to, RINVOQ therapy. Prior to initiating RINVOQ, patients should be brought up to date on all immunizations, including prophylactic varicella zoster or herpes zoster vaccinations, in agreement with current immunization guidelines.
Reports of medication residue in stool or ostomy output have occurred in patients taking RINVOQ. Most reports described anatomic or functional GI conditions with shortened GI transit times. Instruct patients to contact their healthcare provider if medication residue is observed repeatedly. Monitor patients clinically and consider alternative treatment if there is an inadequate therapeutic response.
There are no data on the presence of RINVOQ in human milk, the effects on the breastfed infant, or the effects on milk production. Available data in animals have shown the excretion of RINVOQ in milk. Advise patients that breastfeeding is not recommended during treatment with RINVOQ and for 6 days after the last dose.
RINVOQ is not recommended for use in patients with severe hepatic impairment.
The most common adverse reactions in RINVOQ clinical trials were upper respiratory tract infections, herpes zoster, herpes simplex, bronchitis, nausea, cough, pyrexia, acne, headache, increased blood creatine phosphokinase, hypersensitivity, folliculitis, abdominal pain, increased weight, influenza, fatigue, neutropenia, myalgia, influenza-like illness, elevated liver enzymes, rash, and anemia.
Inform patients that retinal detachment has been reported in clinical trials with RINVOQ. Advise patients to immediately inform their healthcare provider if they develop any sudden changes in vision while receiving RINVOQ.
Dosage Forms and Strengths: RINVOQ is available in 15 mg, 30 mg, and 45 mg extended-release tablets. RINVOQ LQ is available in a 1 mg/mL oral solution.
*Unless otherwise stated, “RINVOQ” in the IMPORTANT SAFETY INFORMATION refers to RINVOQ and RINVOQ LQ.

RA patients met ACR20 at Week 12 or 14 (Primary Endpoints) and Disease Control through Remission
(DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years. Explore the Data
RA patients met ACR20 at Week 12 or 14 (Primary Endpoints) and Disease Control through Remission (DAS28-CRP <2.6)* at Weeks 12 or 14 and observed up to 5 years. Explore the Data
*Clinical remission does not mean drug-free remission or complete absence of disease activity
ACR response criteria incorporate physician and patient assessments, as well as acute inflammatory, pain, and physical function measures.
ACR response criteria incorporate physician and patient assessments, as well as acute inflammatory, pain, and physical function measures.
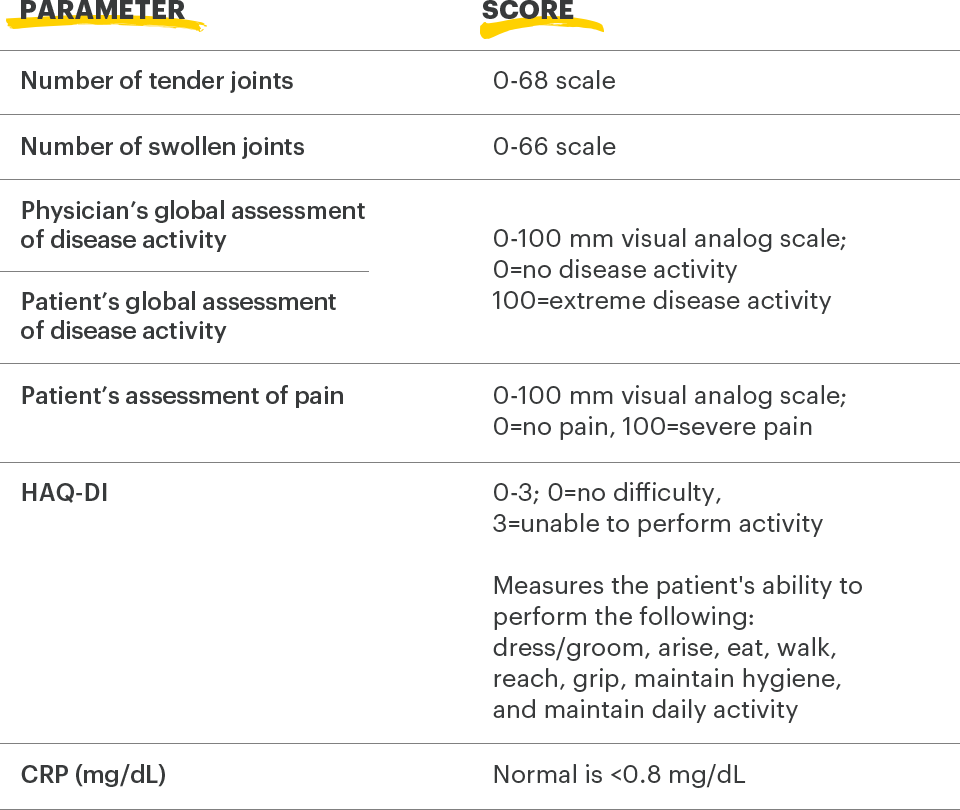

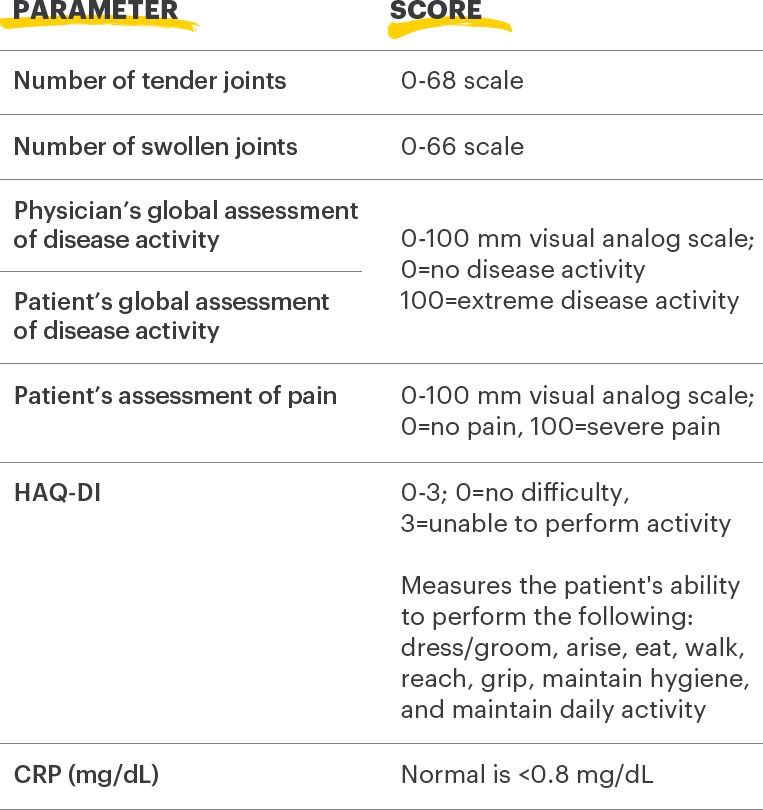
ACR=American College of Rheumatology; CRP=C-reactive protein; HAQ‑Dl=health assessment questionnaire disability index
1. Felson DT, Anderson JJ, Boers M, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729-740.
2. Uhlig T, Haavardsholm EA, Kvien TK. Comparison of the Health Assessment Questionnaire (HAQ) and the modified HAQ (MHAQ) in patients with rheumatoid arthritis. Rheumatology. 2006;45(4):454‑458.
Adults with moderately to severely active RA and inadequate response or intolerance to bDMARDs. 1
RINVOQ is indicated for TNFi-IR patients
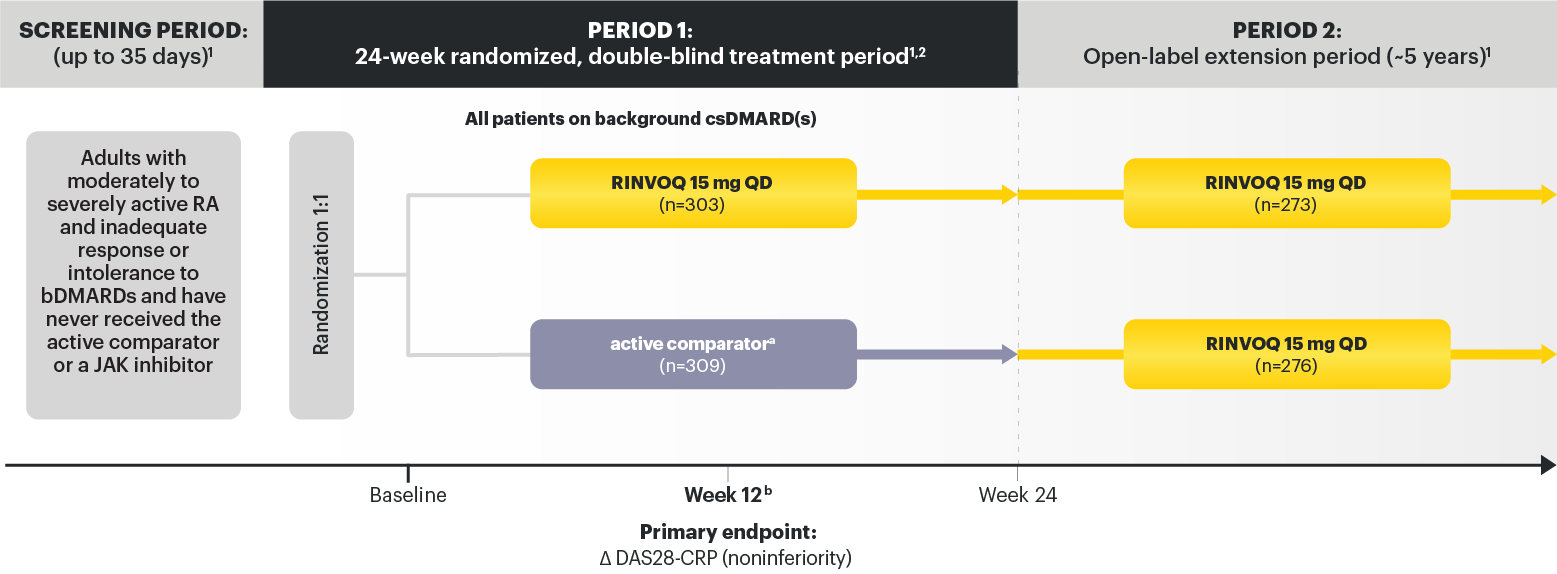

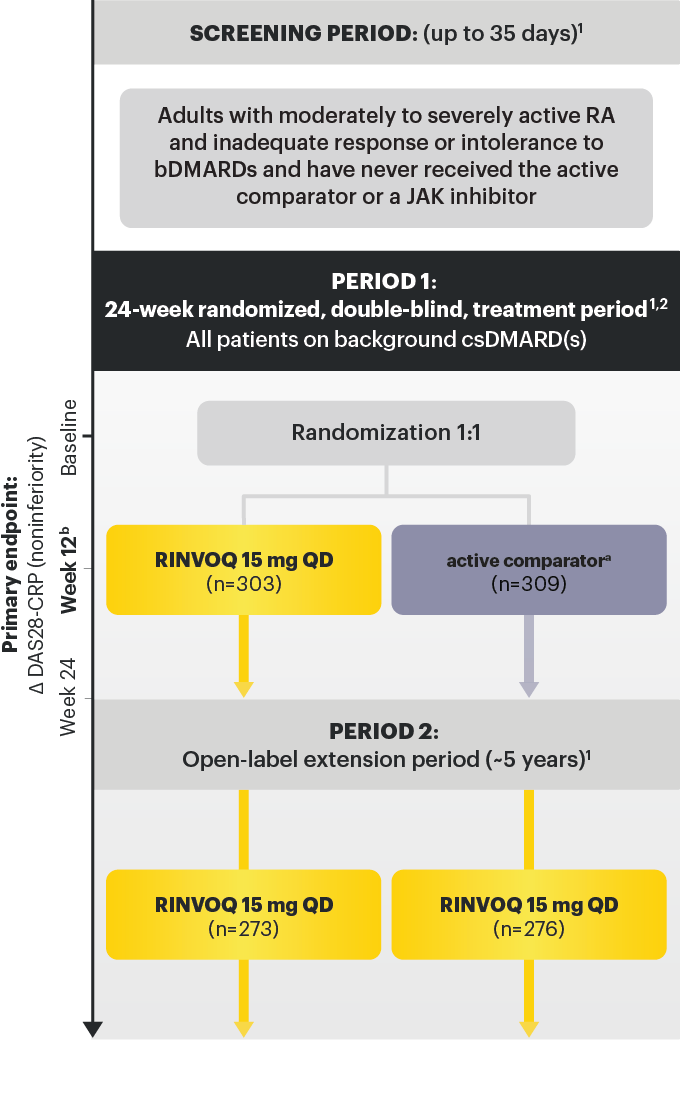
a After the initial dose, active comparator ® IV was administered at 2 and 4 weeks, then every 4 weeks thereafter. The last dose occurred at Week 20. Weight‑based dosing was as follows: 100 kg: 1000 mg. 2
b Starting at Week 12, patients who did not achieve ≥20% improvement in both TJC and SJC compared to baseline at 2 consecutive visits were to have background medication(s) (corticosteroids, NSAIDs, acetaminophen or adding or increasing doses in csDMARD(s)) adjusted or added. 1,3
Data not labeled as a ranked secondary endpoint were prespecified nonranked endpoints not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
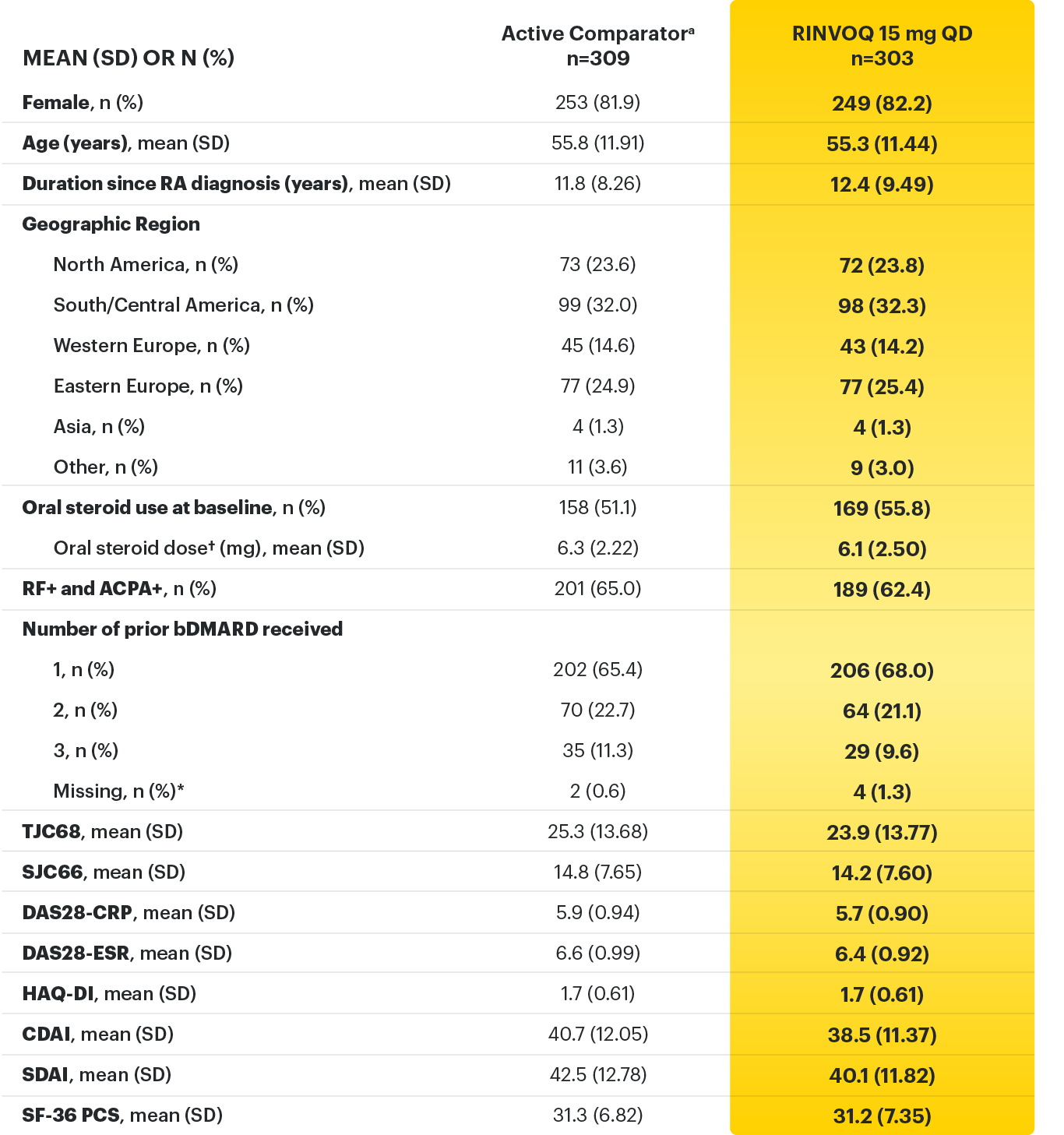

*Did not receive any of the protocol-specified bDMARDs prior to entry.
† Based on prednisone equivalent.
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD=biological disease-modifying anti-rheumatic drug; CDAI=clinical disease activity index; CR=clinical remission; CRP=C-reactive protein; DAS28-CRP=28 joint disease score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; EULAR=European League Against Rheumatism; IV=intravenous; LDA=low disease activity; QD=once per day; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints
1. Rubbert‑Roth A, Enejosa J, Pangan AL, et al. Efficacy and Safety of Upadacitinib vs Abatacept in Patients With Active Rheumatoid Arthritis and Prior Inadequate Response or Intolerance to Biologic Disease‑Modifying Anti‑Rheumatic Drugs (SELECT‑CHOICE): A Double‑Blind, Randomized Controlled Phase 3 Trial. Poster presented at: The European Congress of Rheumatology, 3‑6 June 2020, E‑Congress.
2. Rubbert-Roth A, Enejosa J, Pangan AL, et al. Efficacy and Safety of Upadacitinib vs Abatacept in Patients with Active Rheumatoid Arthritis and Prior Inadequate Response or Intolerance to Biologic Disease‑Modifying Anti‑Rheumatic Drugs (SELECT‑CHOICE): A Double‑Blind, Randomized Controlled Phase 3 Trial. Ann Rheum Dis 2020,79(Supp 1):1011.
3. Data on File. ABVRRTI70957.
4. Data on File. ABVRRTI71850.
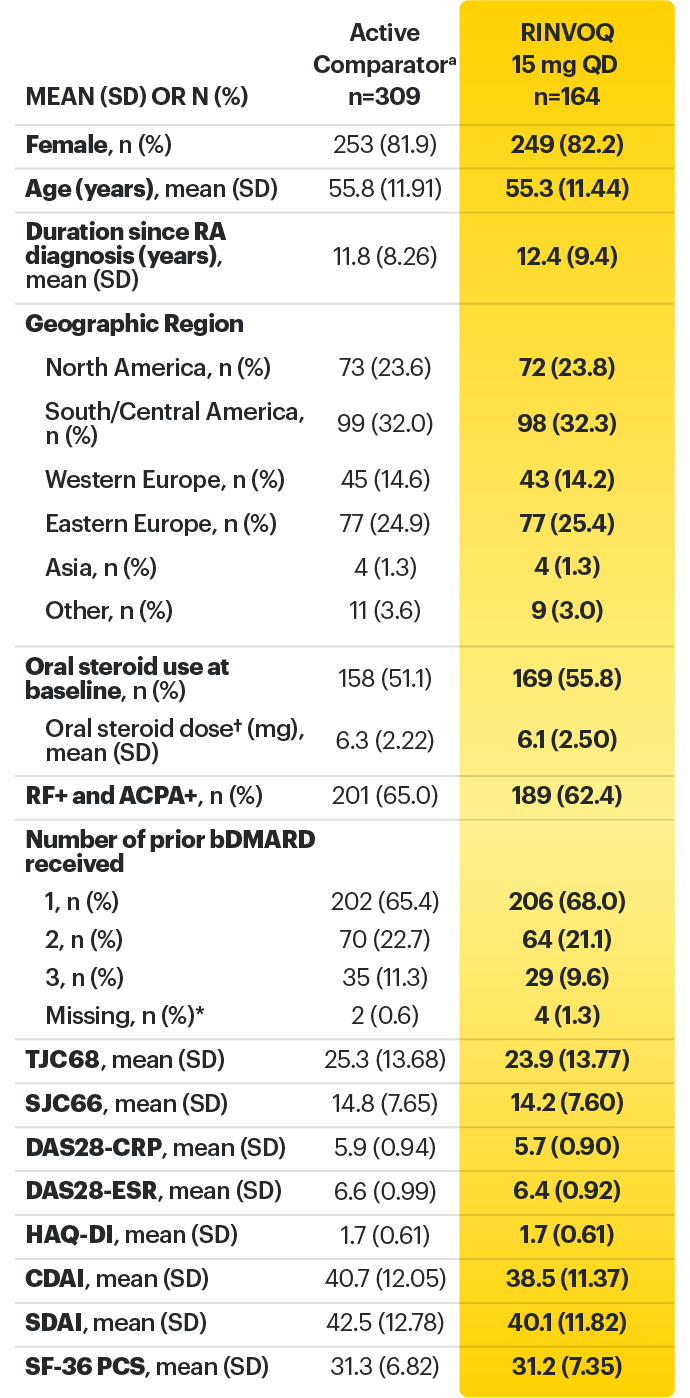
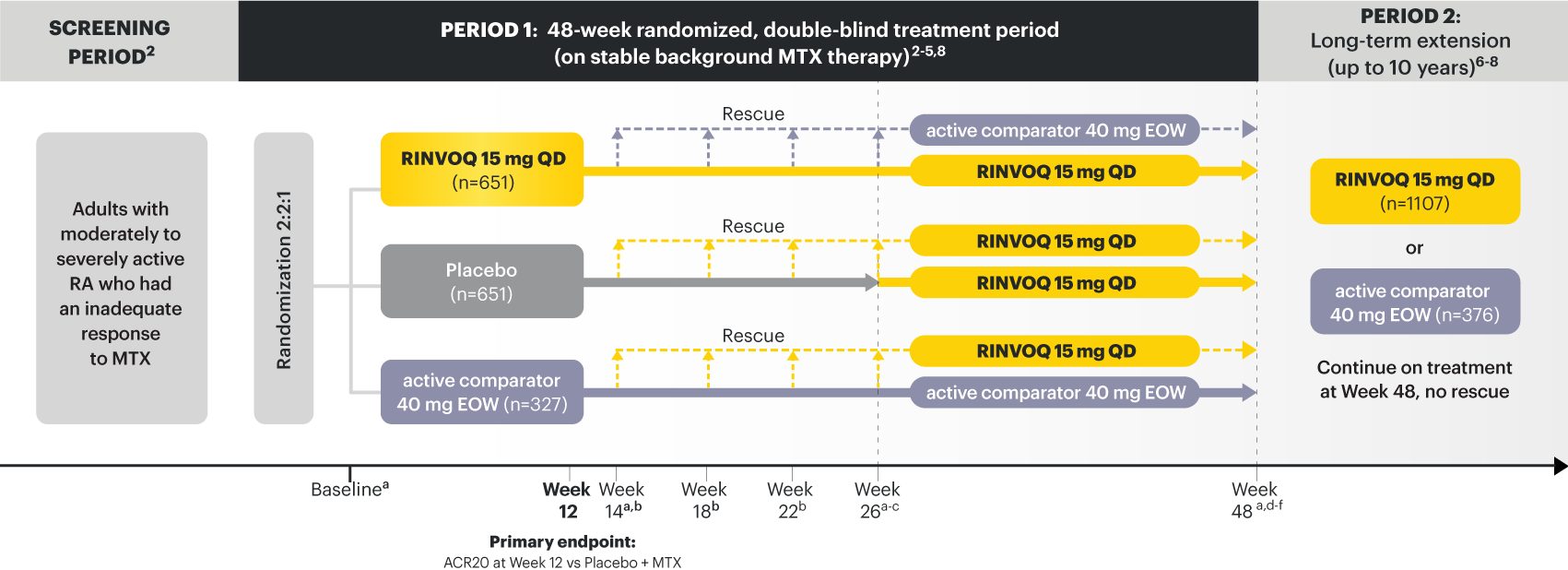
Adults with moderately to severely active RA who had an inadequate response to MTX 1
RINVOQ is indicated for TNFi-IR patients

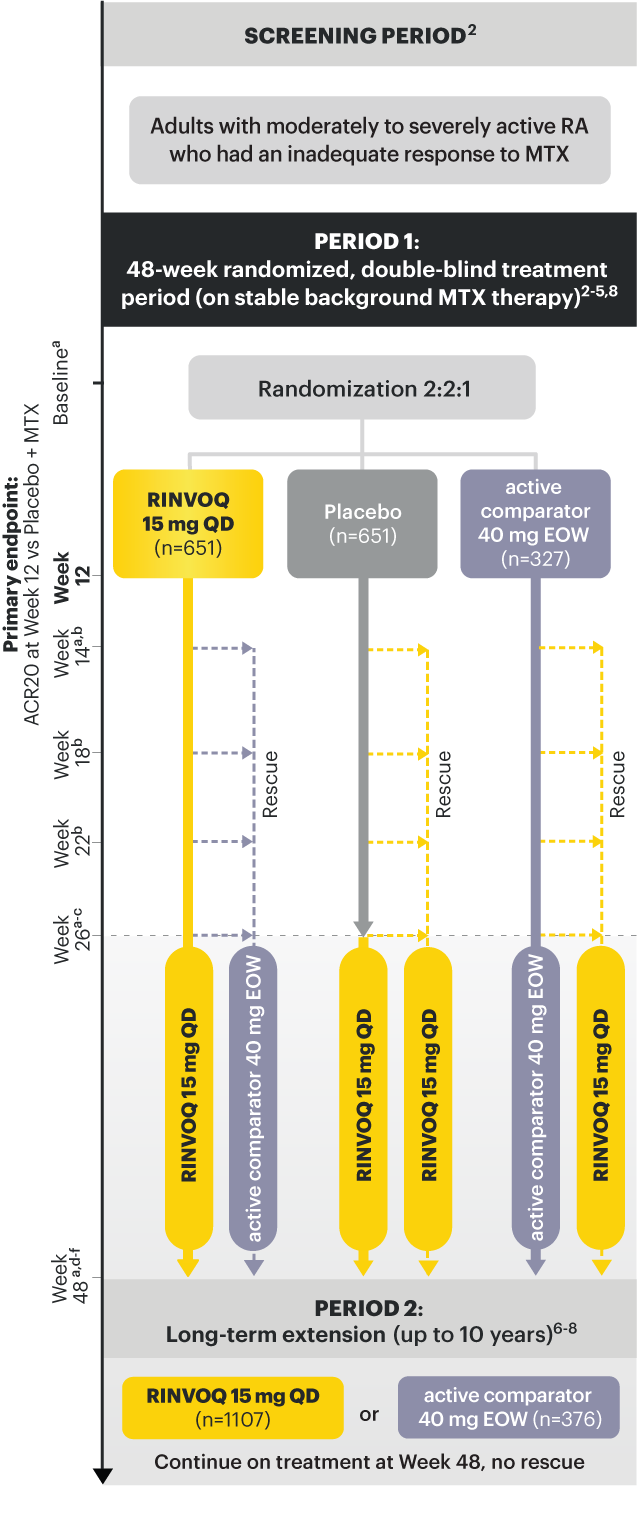
a X-ray imaging was performed at these time points; Week 14 for non-responder patients, who were rescued. 2,3
b Rescue criteria: At Weeks 14, 18 and 22 if 10. 2
c Starting at Week 26, initiation or change in background RA medication(s) including corticosteroids, NSAIDs, or acetaminophen was permitted. 4
d Starting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 5
e At Week 48, initiation or change in csDMARDs was allowed, however not all patients received background MTX. 5
f Patients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter. 8
At Week 12 vs Placebo + MTX:
At Week 26 vs Placebo + MTX:
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs Placebo + MTX. No conclusions regarding this comparison can be made.

*Based on prednisone equivalent
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARDs=biologic disease‑modifying antirheumatic drugs; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C‑reactive protein; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; PBO=Placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Fleischmann R, Pangan AL, Song I-H, et al. Supplement - Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double‑blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788‑1800.
3. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double‑blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788‑1800.
4. Data on File. ABVRRTI70534.
5. Data on File. ABVRRTI70955.
6. Data on File. ABVRRTI68439.
7. Upadacitinib meets all primary and ranked secondary endpoints including superiority versus adalimumab in phase 3 study in rheumatoid arthritis. AbbVie Inc. https://news.abbvie.com/news/upadacitinib-meets-all-primary-and-ranked-secondary-endpoints-including-superiority-versus-adalimumab-in-phase-3-study-in-rheumatoid-arthritis.htm. April 9, 2018. August 15, 2019.
8. Fleishmann R, Mysler E, Bessette L, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis results at 3 years from the SELECT-COMPARE study. Poster presented at: the American College of Rheumatology Convergence, 5-9 November 2021. E-Congress.
9. Data On File. ABVRRTI68440.
10. Data on File. ABVRRTI70956.
11. Fleischmann R, Pangan AL, Mysler E, et al. A phase 3, randomized, double‑blind study comparing upadacitinib to placebo and to adalimumab, in patients with active rheumatoid arthritis with inadequate response to methotrexate. Oral presentation at: The ACR annual meeting 2018, 19–24 October 2018, Chicago, USA.
Adults with moderately to severely active RA who had an inadequate response to MTX 1
RINVOQ is indicated for TNFi-IR patients

Upadacitinib 30 mg is not an approved dose.
a Patients on MTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14. 3
b Starting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDS, acetaminophen or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 5
c Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD including those previously on UPA 30 mg. 7
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.

*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.
† Prednisone equivalent
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=clinical disease activity index; cMTX=continuous methotrexate; csDMARDs=conventional synthetic disease‑modifying antirheumatic drugs; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C‑reactive protein; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Smolen JS, Pangan AL, Emery P, et al. Supplement - Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT‑MONOTHERAPY): a randomised, placebo‑controlled, double‑blind phase 3 study. Lancet. 2019;393(10188):2303-2311.
3. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT‑MONOTHERAPY): a randomised, placebo‑controlled, double‑blind phase 3 study. Lancet. 2019;393(10188):2303-2311.
4. Smolen JS, Emery P, Rigby W, et al. Upadacitinib as monotherapy in patients with rheumatoid arthritis: results at 48 weeks from the SELECT‑MONOTHERAPY study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
5. Data on File. ABVRRTI68979.
6. Data on File. ABVRRTI68841.
7. A study comparing upadacitinib (ABT-494) monotherapy to methotrexate (MTX) monotherapy in adults with rheumatoid arthritis (RA) who have an inadequate response to MTX (SELECT-MONOTHERAPY). ClinicalTrials.gov identifier: NCT02706951. https://clinicaltrials.gov/ct2/show/NCT02706951. Updated August 2, 2021. Accessed November 30, 2021.
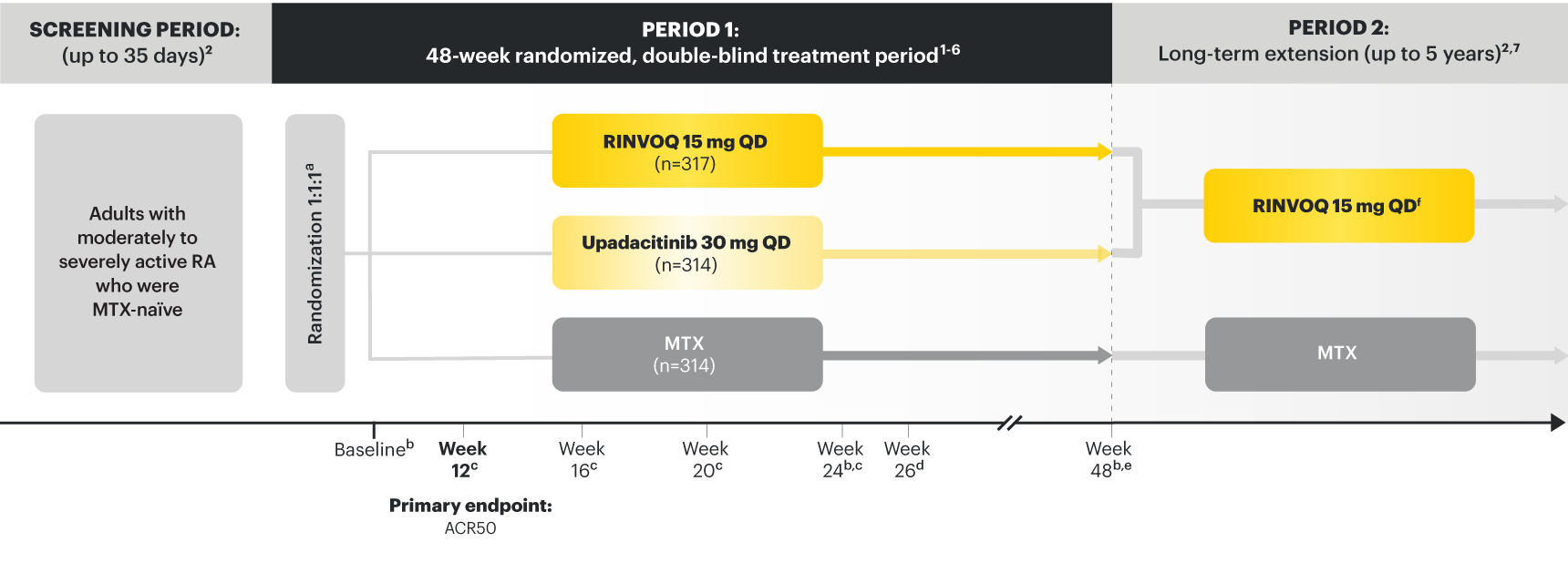
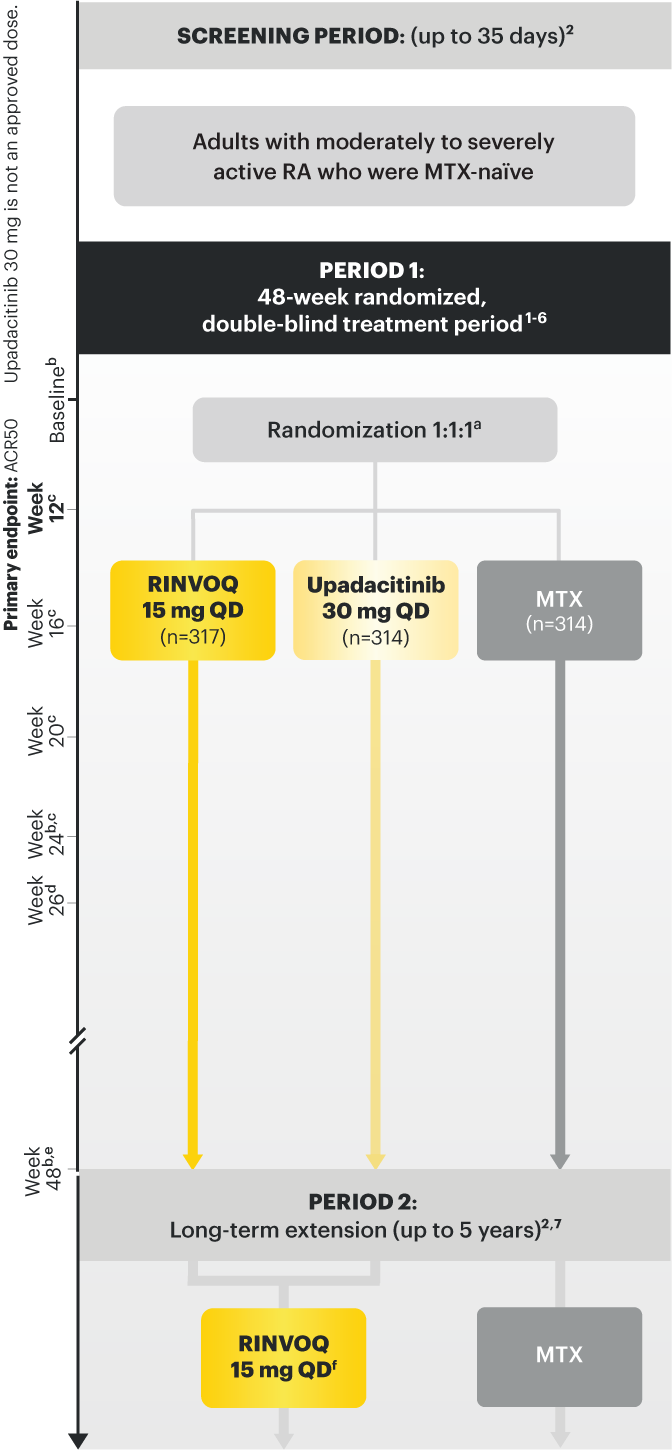
Adults with moderately to severely active RA who were MTX‑naïve 1
RINVOQ is indicated for TNFi-IR patients

Upadacitinib 30 mg is not an approved dose.
a Initially 947 patients were randomized in the study, but two patients were never dosed. 2
b X-ray images of hands and feet obtained at these time points. 2
c Starting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at two consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics). 3,4
d At Week 26, patients with CDAI≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI>2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and e Starting at Week 48, patients who did not achieve ≥ 20% improvement in both TJC and SJC at two consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2. 6
f Following a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received UPA 15 mg. 11
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.

*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA = anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=clinical disease activity index; CR=clinical remission; CRP=C reactive protein; csDMARDs=conventional synthetic disease-modifying antirheumatic drugs; DAS28-CRP=28 joint disease activity score using C-reactive protein; HAQ-DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C-reactive protein; JE=joint erosion score; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Van Vollenhoven R, Takeuchi T, Pangan AL, et al. Supplement: Efficacy and safety of upadacitinib monotherapy in methotrexate‑naïve patients with moderately to severely active rheumatoid arthritis (SELECT‑EARLY): a randomized, double-blind, active‑comparator, multi‑center, multi‑country trial. Arthritis Rheumatol. 2020. doi:10.1002/art.41384.
3. Van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate‑naïve patients with moderately to severely active rheumatoid arthritis (SELECT‑EARLY): a randomized, double‑blind, active‑comparator, multi-center, multi‑country trial. Arthritis Rheumatol. 2020. doi:10.1002/art.41384.
4. Data on File. ABVRRTI70661.
5. Data on File. ABVRRTI68563.
6. Data on File. ABVRRTI70953.
7. Van Vollenhoven R, Takeuchi T, Rischmueller M, et al. Upadacitinib monotherapy in methotrexate‑naïve patients with rheumatoid arthritis: results at 72 weeks from SELECT‑EARLY. Poster presented at: The European Congress of Rheumatology, 3‑6 June 2020, E-Congress.
8. Data on File. ABVRRTI68564.
9. Data on File. ABVRRTI70539.
10. Data on File. ABVRRTI70954.
11. A study to compare upadacitinib (ABT-494) monotherapy to methotrexate (MTX) monotherapy in adults with rheumatoid arthritis (RA) who have not previously taken methotrexate (SELECT-EARLY). ClinicalTrials.gov identifier: NCT02706873. https://clinicaltrials.gov/ct2/show/NCT02706873. Updated August 2, 2021. Accessed November 30, 2021.
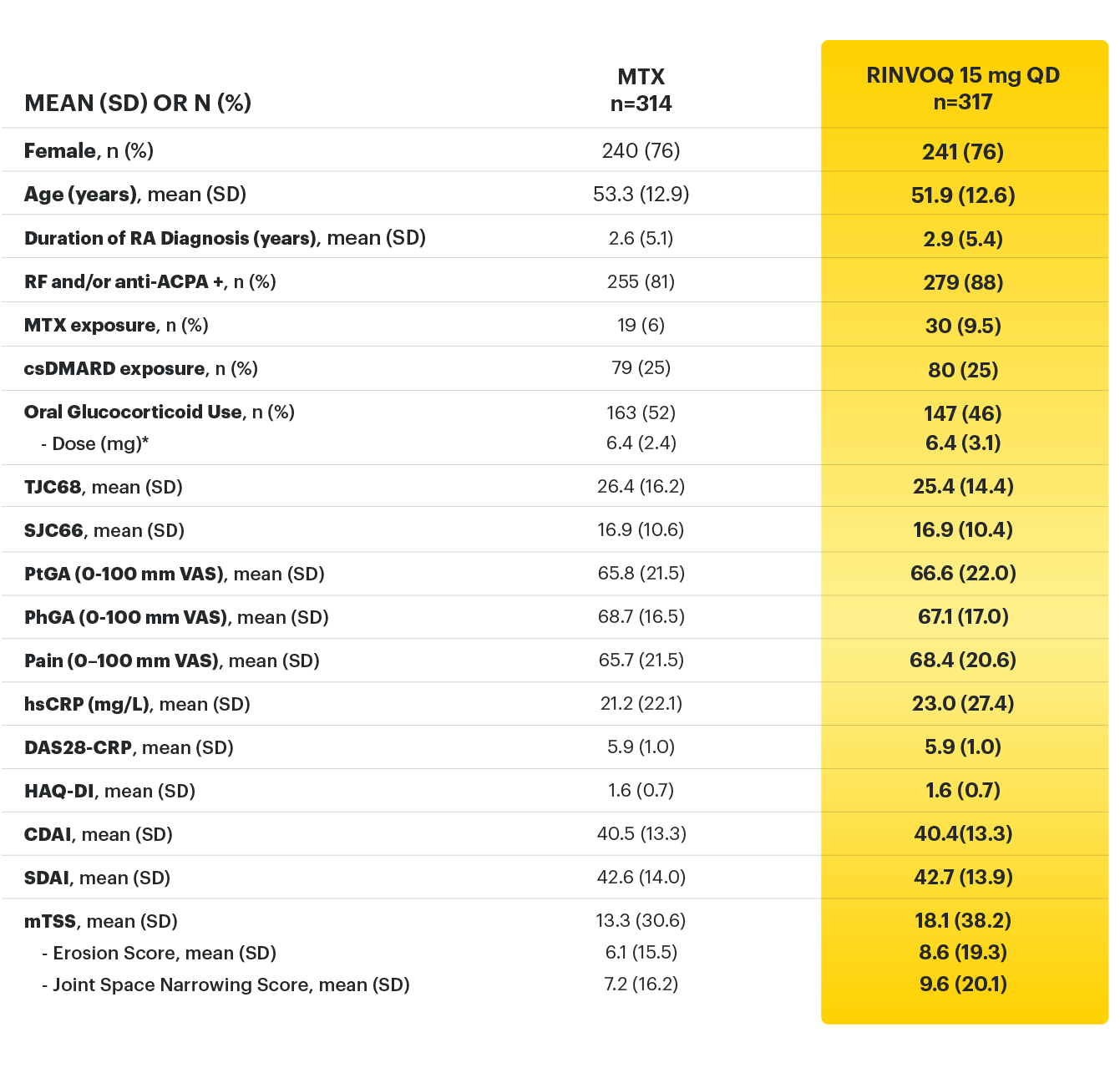
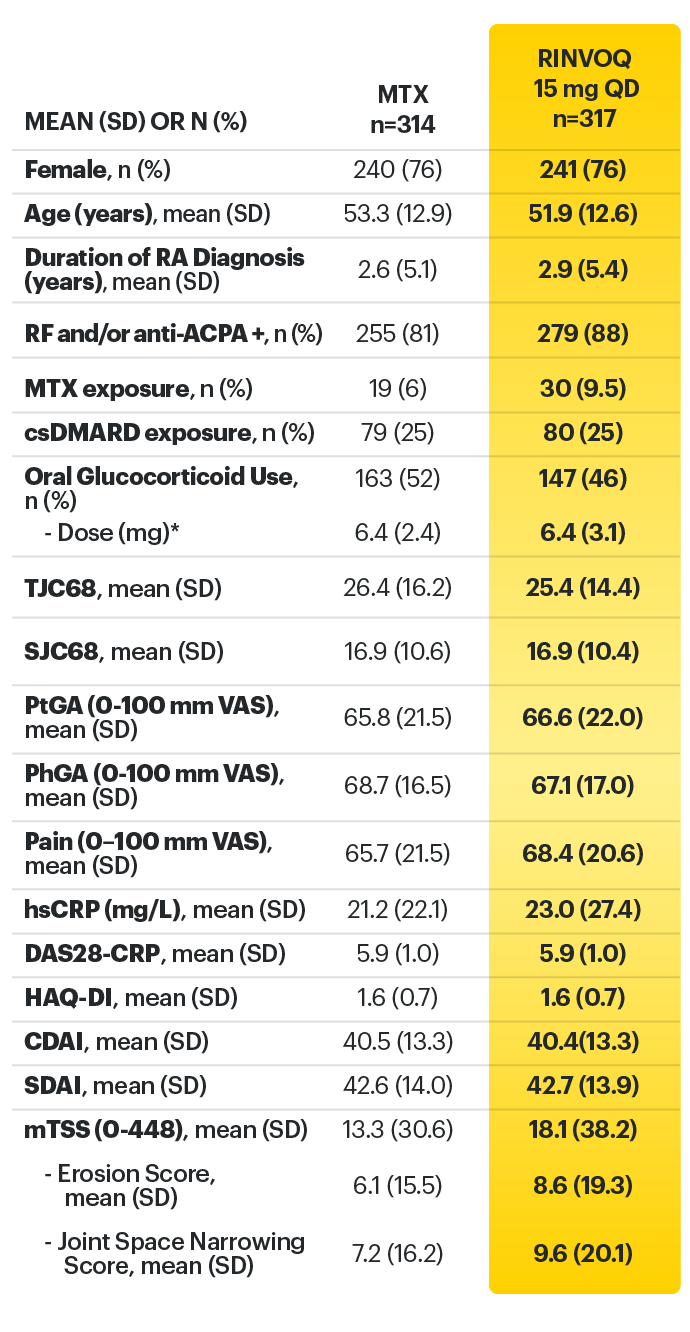
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s) 1
RINVOQ is indicated for TNFi-IR patients
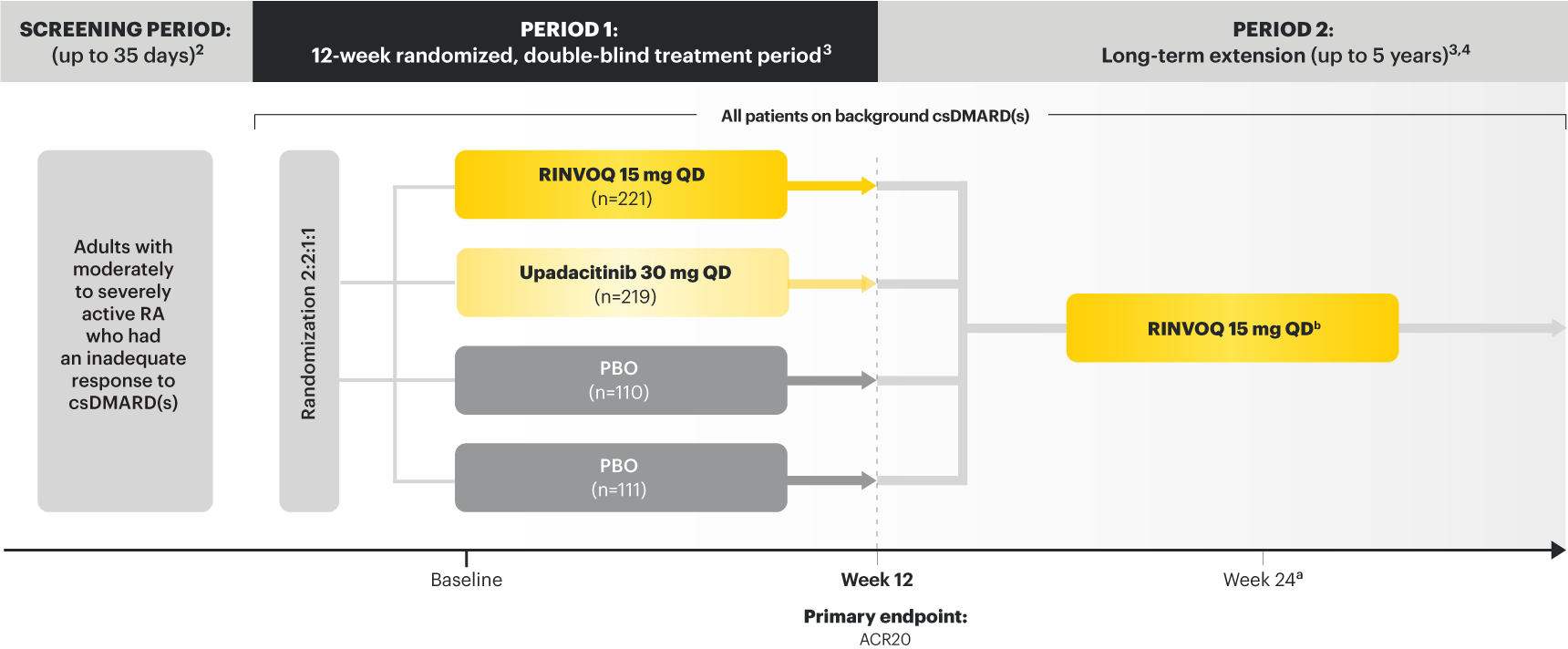

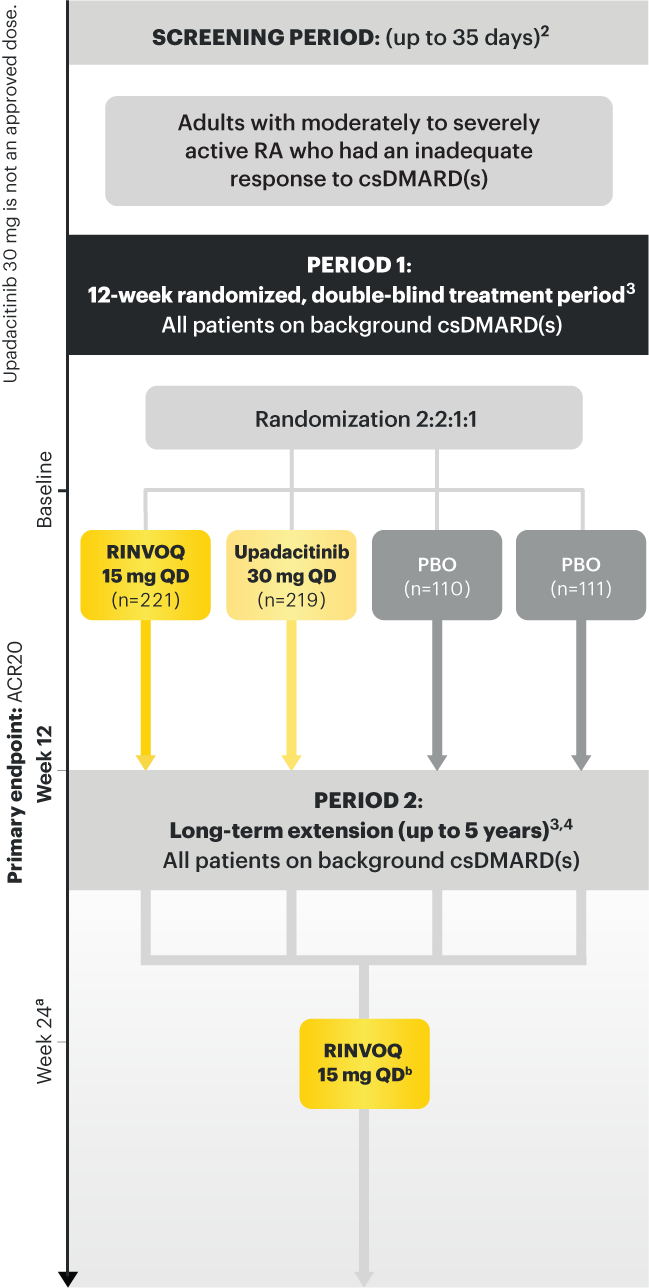
Upadacitinib 30 mg is not an approved dose.
a Starting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDS, acetaminophen or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 4
b Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg. 7
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.

*Based on prednisone equivalent
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD= biologic disease‑modifying antirheumatic drug; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying anti-rheumatic drug; DAS28-CRP=28 joint disease activity score using C-reactive protein; HAQ-DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C-reactive protein; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale.
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Burmester GR, Kremer JM, Van den Bosch F, et al. Supplement - Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‑modifying anti‑rheumatic drugs (SELECT‑NEXT): a randomised, double‑blind, placebo‑controlled phase 3 trial. Lancet . 2018;391(10139):2503‑2512.
3. Burmester GR, Van den Bosch F, Bessette L, et al. Long‑term safety and efficacy of upadacitinib in patients with rheumatoid arthritis and an inadequate response to csDMARDs: results at 60 weeks from the SELECT‑NEXT study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
4. Data on File. ABVRRTI68981.
5. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‑modifying anti‑rheumatic drugs (SELECT‑NEXT): a randomised, double‑blind, placebo‑controlled phase 3 trial. Lancet. 2018;391(10139):2503‑2512.
6. Data on File. ABVRRTI68843.
7. Data On File. ABVRRTI73233.
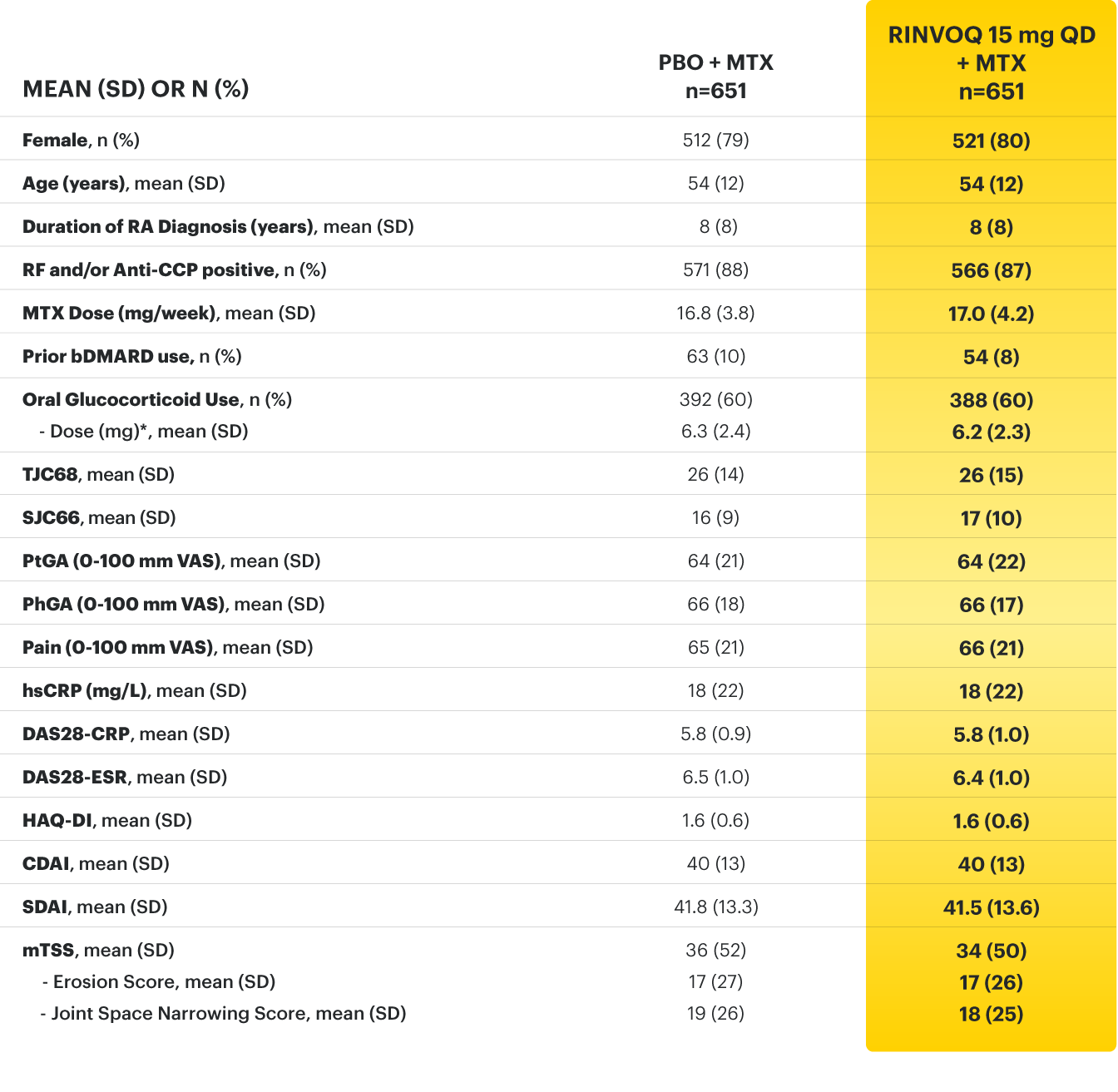
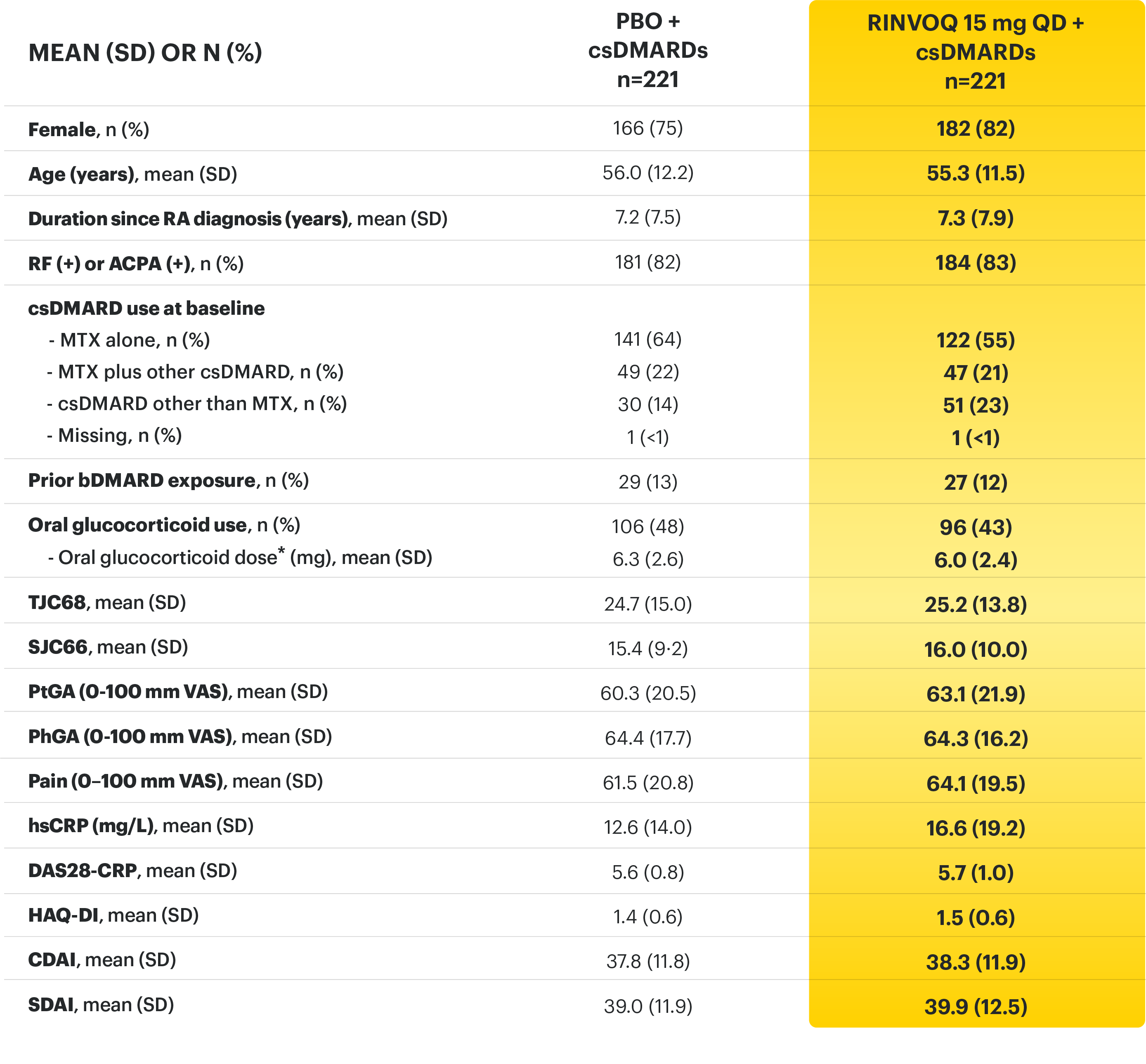
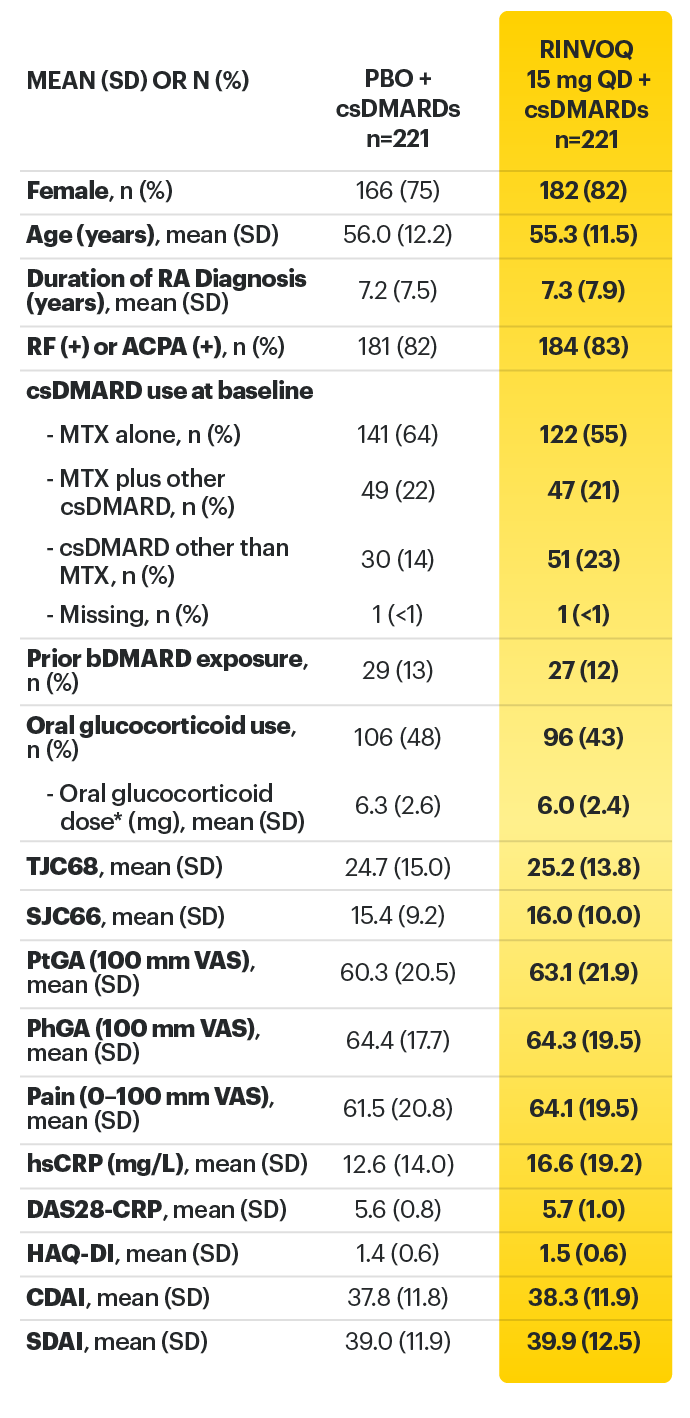
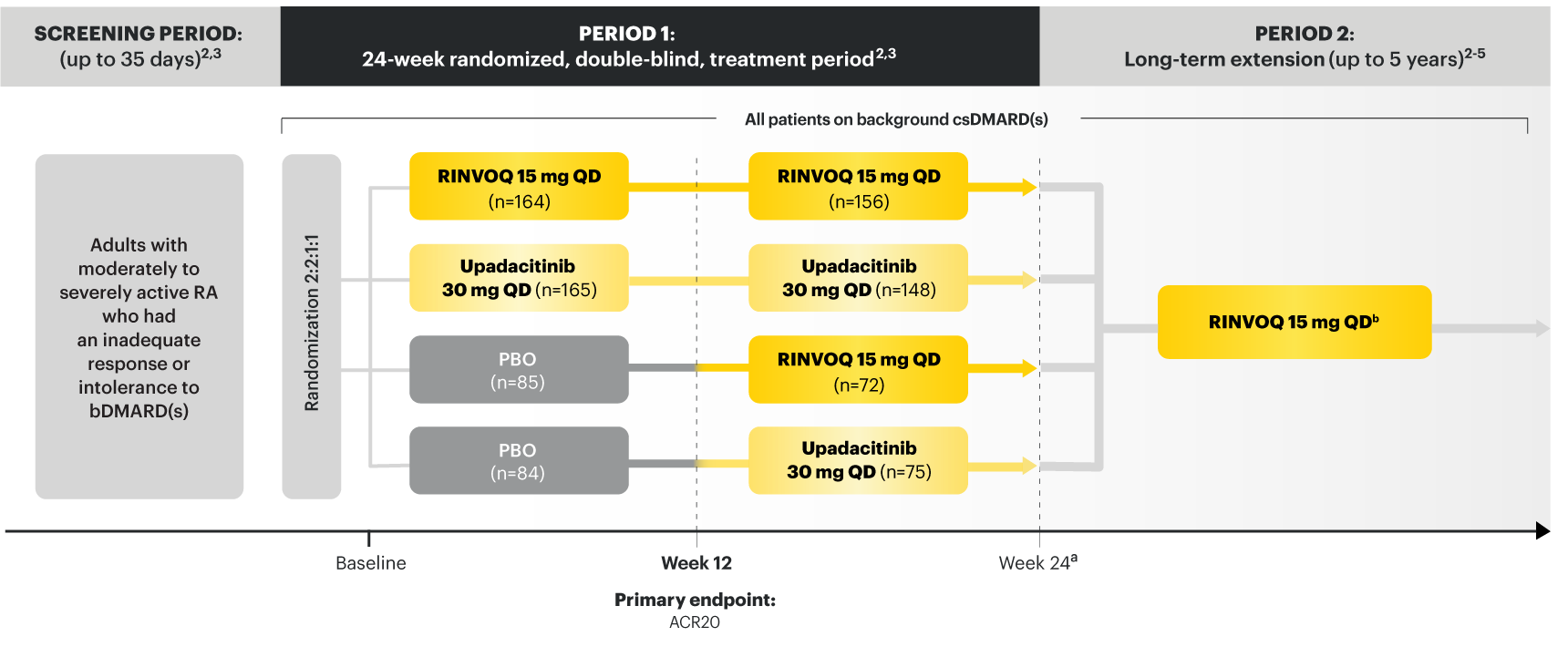
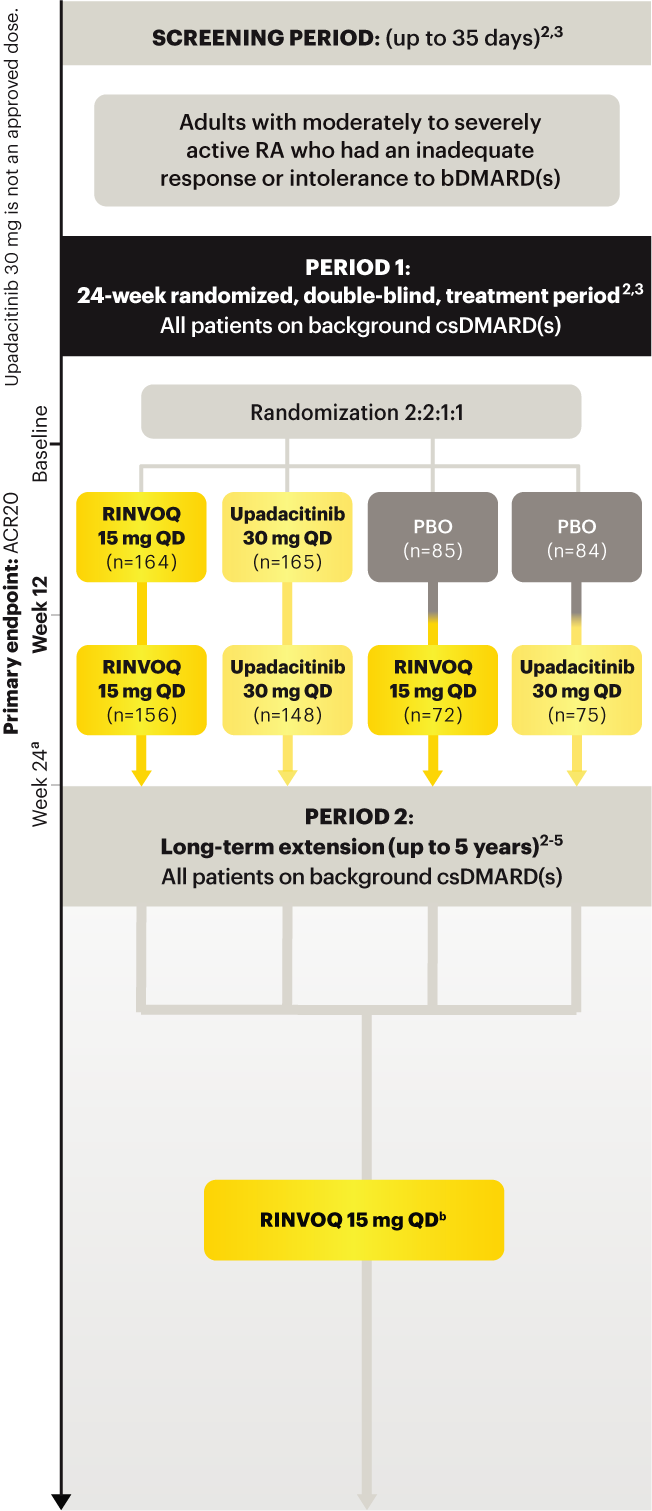
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs 1
RINVOQ is indicated for TNFi-IR patients

Upadacitinib 30 mg is not an approved dose.
a Starting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at two consecutive visits were removed from the study. 4,5
b Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD including those previously on UPA 30 mg. 8
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
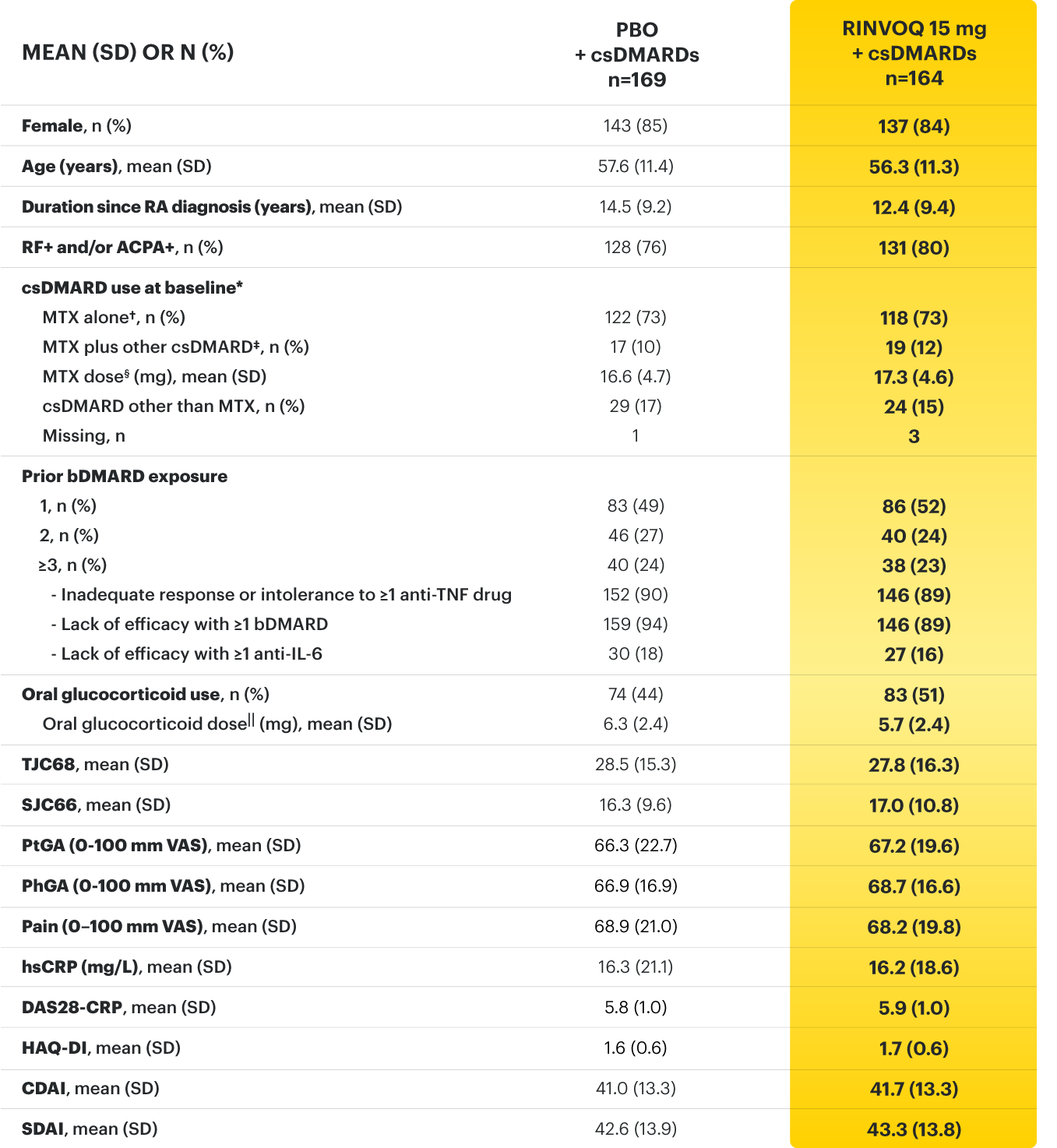

* Oral or parenteral methotrexate (7.5-25 mg per week).
† Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡ All combinations allowed except MTX and leflunomide.
§ Mean MTX dose calculated only for patients receiving MTX.
‖ Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARDs=biologic disease‑modifying antirheumatic drugs; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; hsCRP=high‑sensitivity C‑reactive protein; HAQ‑DI=health assessment questionnaire disability index; IL-6=interleukin 6; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Genovese MC, Fleischmann R, Combe B, et al. Supplement - Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease‑modifying anti‑rheumatic drugs (SELECT‑BEYOND): a double‑blind, randomised controlled phase 3 trial. Lancet . 2018;391(10139):2513‑2524.
3. Genovese MC, Combe B, Hall S, et al. Upadacitinib in patients with rheumatoid arthritis and inadequate response or intolerance to biologic DMARDs: results at 60 weeks from the SELECT‑BEYOND Study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
4. Data on File. ABVRRTI68669.
5. Data on File. ABVRRTI68670.
6. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease‑modifying anti‑rheumatic drugs (SELECT‑BEYOND): a double‑blind, randomised controlled phase 3 trial. Lancet . 2018;391(10139):2513‑2524.
7. Data on File. ABVRRTI68842.
8. A study to compare upadacitinib (ABT-494) to placebo in adults with rheumatoid arthritis on stable dose of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) with an inadequate response or intolerance to biologic DMARDs (SELECT-BEYOND). ClinicalTrials.gov identifier: NCT02706847. https://clinicaltrials.gov/ct2/show/NCT02706847. Updated August 2, 2021. Accessed November 30, 2021.
HUMIRA is indicated, alone or in combination with methotrexate or other non-biologic DMARDs, for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis.
Patients treated with HUMIRA are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue HUMIRA if a patient develops a serious infection or sepsis.
Reported infections include:
Carefully consider the risks and benefits of treatment with HUMIRA prior to initiating therapy in patients: 1. with chronic or recurrent infection, 2. who have been exposed to TB, 3. with a history of opportunistic infection, 4. who resided in or traveled in regions where mycoses are endemic, 5. with underlying conditions that may predispose them to infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with HUMIRA, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including HUMIRA. Postmarketing cases of hepatosplenic T‑cell lymphoma (HSTCL), a rare type of T‑cell lymphoma, have been reported in patients treated with TNF blockers, including HUMIRA. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. Almost all of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants.
HEPATITIS B VIRUS REACTIVATION
CONGESTIVE HEART FAILURE
Reference: 1. HUMIRA Injection [package insert]. North Chicago, IL: AbbVie Inc.
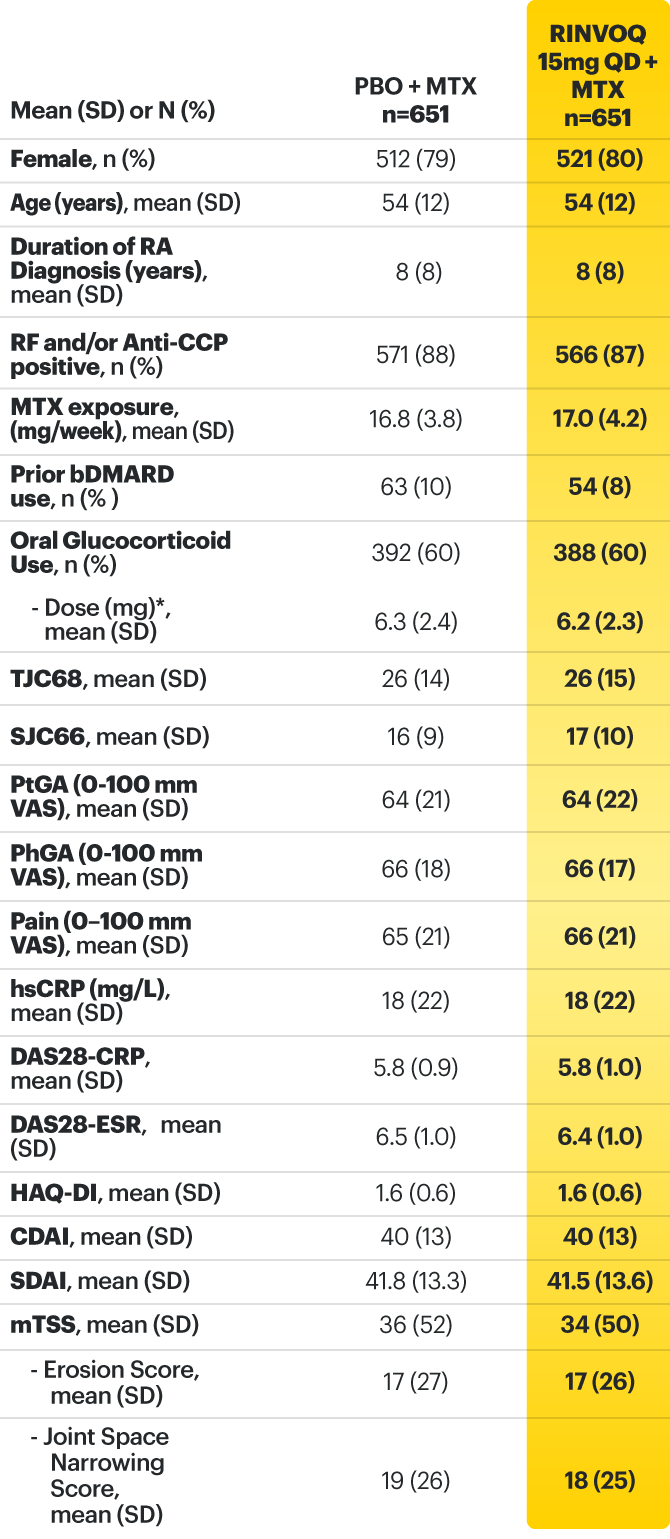
Adults with moderately to severely active RA who had an inadequate response or intolerance to bDMARDs 1
RINVOQ is indicated for TNFi-IR patients



Upadacitinib 30 mg is not an approved dose.
a Starting at Week 24, initiation of or change in corticosteroids, NSAIDs, acetaminophen, and csDMARDs was permitted. Patients not achieving response criteria ≥20% improvement in SJC and TJC at two consecutive visits were removed from the study. 4,5
b Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD including those previously on UPA 30 mg. 8
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.



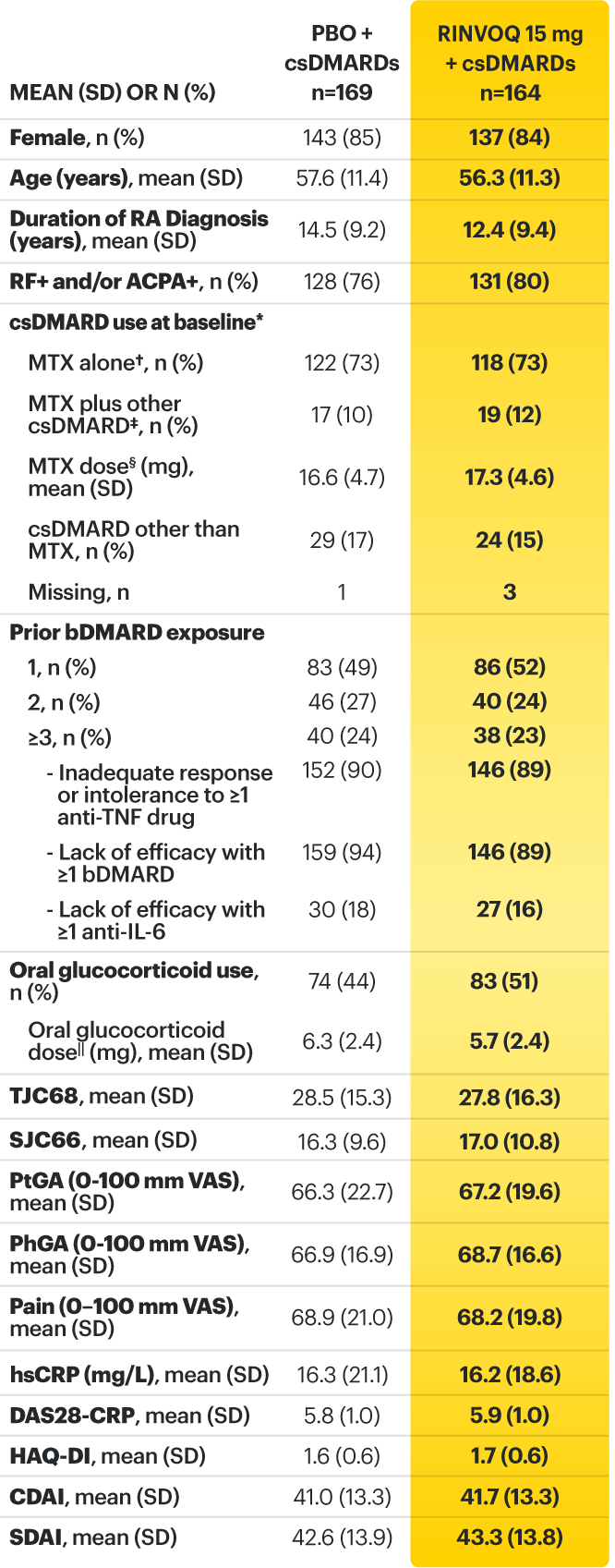
* Oral or parenteral methotrexate (7.5-25 mg per week).
† Data available for 168 patients receiving placebo and 161 patients receiving RINVOQ 15 mg.
‡ All combinations allowed except MTX and leflunomide.
§ Mean MTX dose calculated only for patients receiving MTX.
‖ Based on prednisone equivalent.
ACPA=anti‑citrullinated protein antibodies; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARDs=biologic disease‑modifying antirheumatic drugs; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease‑modifying antirheumatic drug; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; hsCRP=high‑sensitivity C‑reactive protein; HAQ‑DI=health assessment questionnaire disability index; IL-6=interleukin 6; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36‑item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; TNFi=tumor necrosis factor inhibitor; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Genovese MC, Fleischmann R, Combe B, et al. Supplement - Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease‑modifying anti‑rheumatic drugs (SELECT‑BEYOND): a double‑blind, randomised controlled phase 3 trial. Lancet . 2018;391(10139):2513‑2524.
3. Genovese MC, Combe B, Hall S, et al. Upadacitinib in patients with rheumatoid arthritis and inadequate response or intolerance to biologic DMARDs: results at 60 weeks from the SELECT‑BEYOND Study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
4. Data on File. ABVRRTI68669.
5. Data on File. ABVRRTI68670.
6. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease‑modifying anti‑rheumatic drugs (SELECT‑BEYOND): a double‑blind, randomised controlled phase 3 trial. Lancet . 2018;391(10139):2513‑2524.
7. Data on File. ABVRRTI68842.
8. A study to compare upadacitinib (ABT-494) to placebo in adults with rheumatoid arthritis on stable dose of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) with an inadequate response or intolerance to biologic DMARDs (SELECT-BEYOND). ClinicalTrials.gov identifier: NCT02706847. https://clinicaltrials.gov/ct2/show/NCT02706847. Updated August 2, 2021. Accessed November 30, 2021.
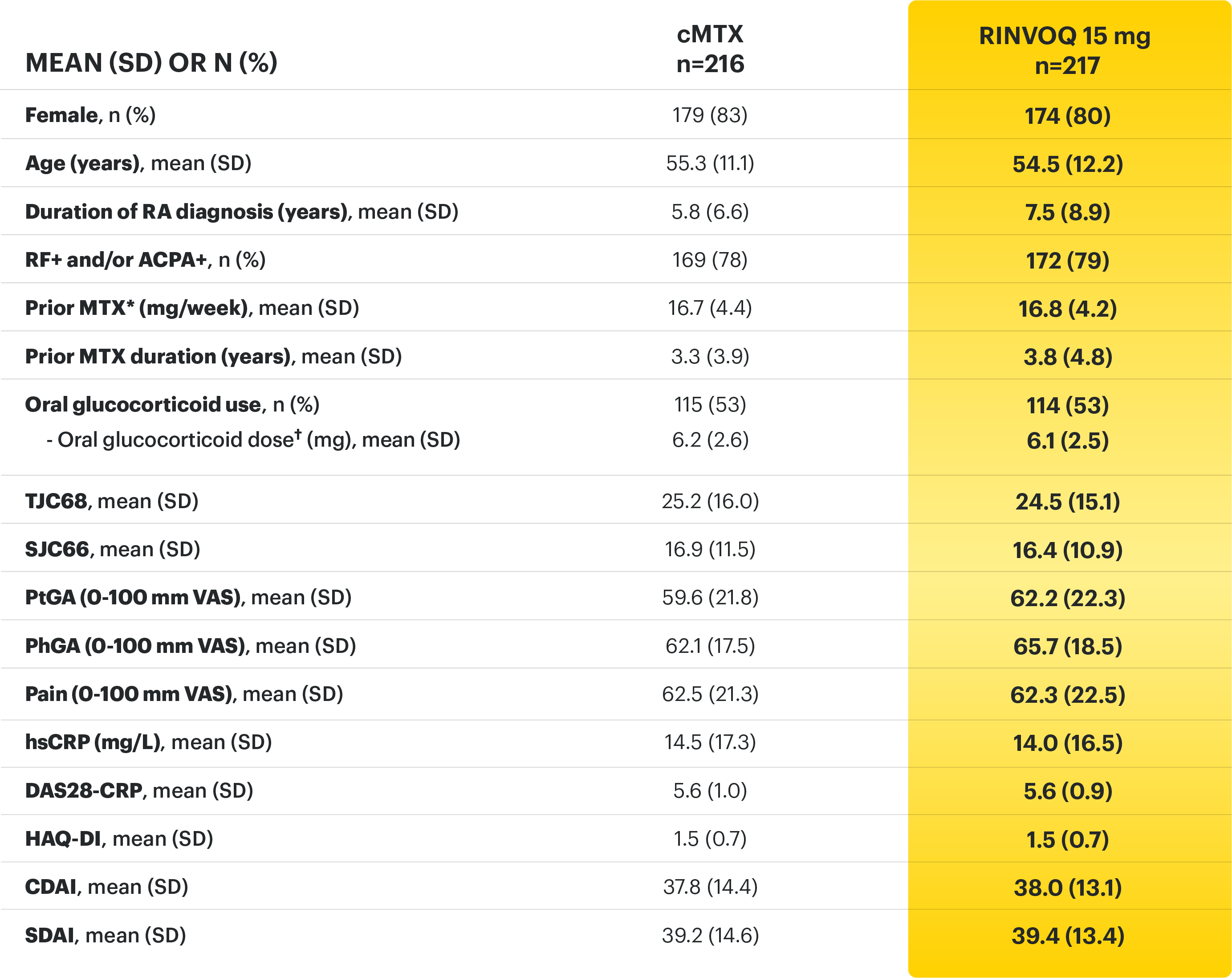
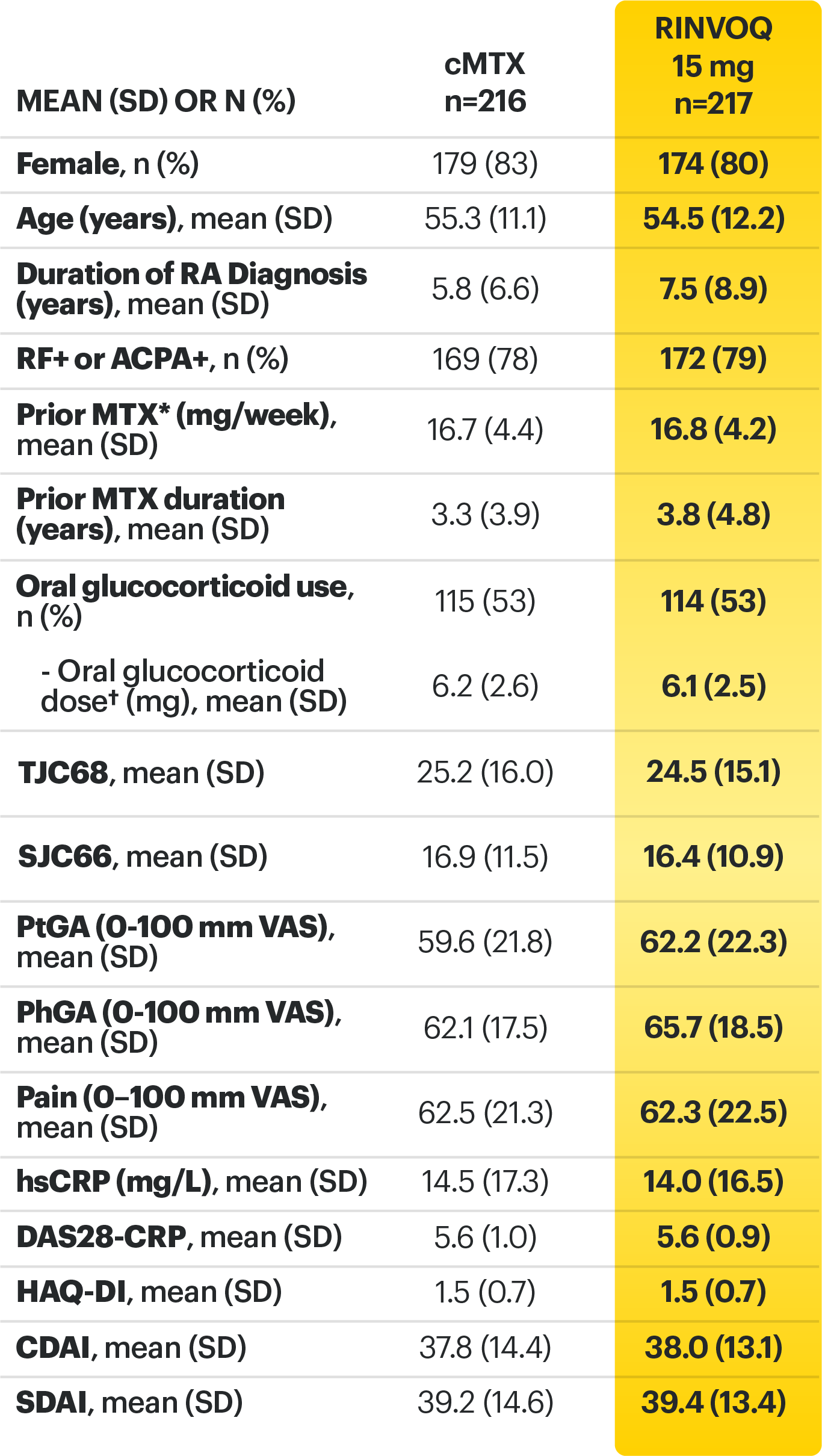
Adults with moderately to severely active RA who were MTX‑naïve 1
RINVOQ is indicated for TNFi-IR patients



Upadacitinib 30 mg is not an approved dose.
a Initially 947 patients were randomized in the study, but two patients were never dosed. 2
b X-ray images of hands and feet obtained at these time points. 2
c Starting at Week 12, patients with ≤20% improvement in TJC and SJC compared to baseline at two consecutive visits continued blinded therapy and optimized background RA medications (corticosteroids, NSAIDs, and/or low‑potency analgesics). 3,4
d At Week 26, patients with CDAI≤2.8 continued their original study drug; background medications (NSAIDs, corticosteroids, and/or low‑potency analgesics, and csDMARDs) were optimized in patients with CDAI>2.8 but ≥20% improvement in TJC and SJC; among patients with CDAI >2.8 and e Starting at Week 48, patients who did not achieve ≥ 20% improvement in both TJC and SJC at two consecutive visits were removed from the study. Initiation of or change in background RA medications (NSAIDs, corticosteroids, low potency analgesics, and csDMARDs; not all patients received background MTX) is allowed at anytime during Period 2. 6
f Following a protocol amendment, all patients in the long-term extension who were previously receiving UPA 30 mg received UPA 15 mg. 11
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.



*Prednisone equivalent dose in patients receiving oral glucocorticoids at baseline.
ACPA = anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=clinical disease activity index; CR=clinical remission; CRP=C reactive protein; csDMARDs=conventional synthetic disease-modifying antirheumatic drugs; DAS28-CRP=28 joint disease activity score using C-reactive protein; HAQ-DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C-reactive protein; JE=joint erosion score; JSN=joint space narrowing; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; NSAIDs=nonsteroidal anti-inflammatory drugs; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF-36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Van Vollenhoven R, Takeuchi T, Pangan AL, et al. Supplement: Efficacy and safety of upadacitinib monotherapy in methotrexate‑naïve patients with moderately to severely active rheumatoid arthritis (SELECT‑EARLY): a randomized, double-blind, active‑comparator, multi‑center, multi‑country trial. Arthritis Rheumatol. 2020. doi:10.1002/art.41384.
3. Van Vollenhoven R, Takeuchi T, Pangan AL, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate‑naïve patients with moderately to severely active rheumatoid arthritis (SELECT‑EARLY): a randomized, double‑blind, active‑comparator, multi-center, multi‑country trial. Arthritis Rheumatol. 2020. doi:10.1002/art.41384.
4. Data on File. ABVRRTI70661.
5. Data on File. ABVRRTI68563.
6. Data on File. ABVRRTI70953.
7. Van Vollenhoven R, Takeuchi T, Rischmueller M, et al. Upadacitinib monotherapy in methotrexate‑naïve patients with rheumatoid arthritis: results at 72 weeks from SELECT‑EARLY. Poster presented at: The European Congress of Rheumatology, 3‑6 June 2020, E-Congress.
8. Data on File. ABVRRTI68564.
9. Data on File. ABVRRTI70539.
10. Data on File. ABVRRTI70954.
11. A study to compare upadacitinib (ABT-494) monotherapy to methotrexate (MTX) monotherapy in adults with rheumatoid arthritis (RA) who have not previously taken methotrexate (SELECT-EARLY). ClinicalTrials.gov identifier: NCT02706873. https://clinicaltrials.gov/ct2/show/NCT02706873. Updated August 2, 2021. Accessed November 30, 2021.
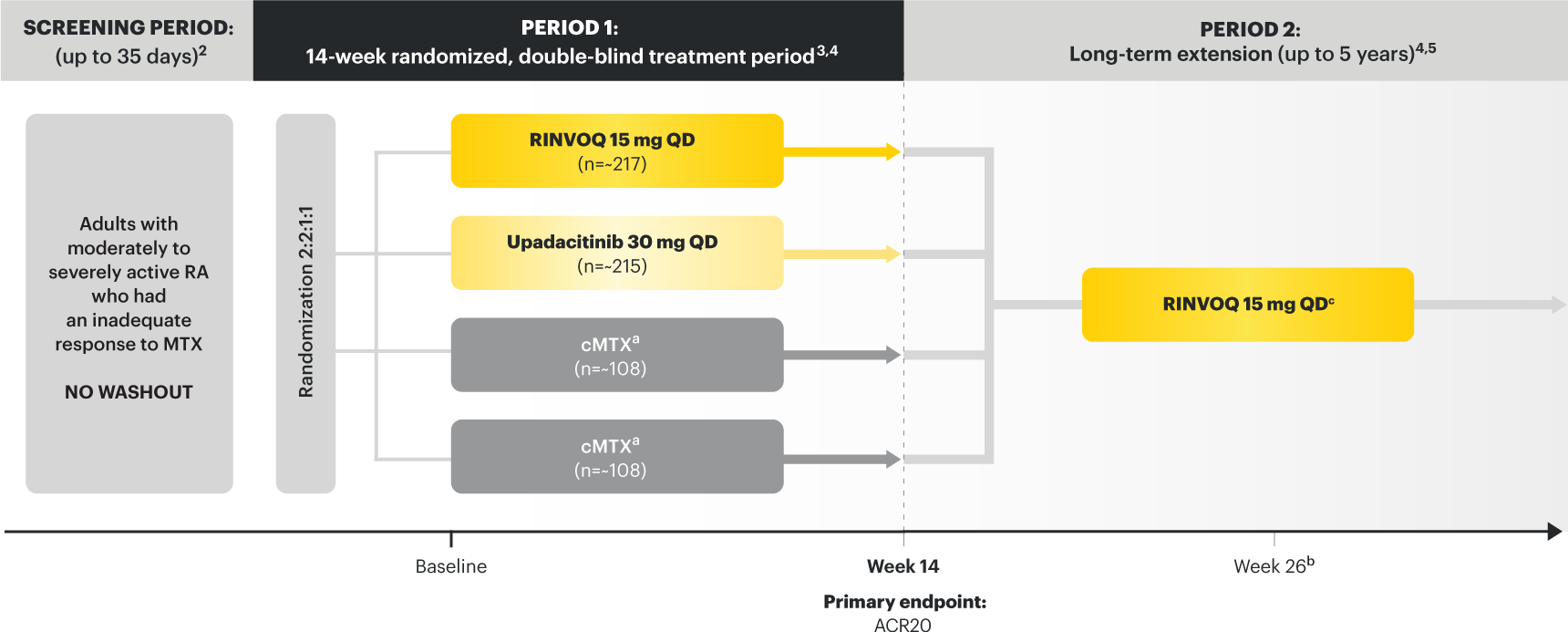
Adults with moderately to severely active RA who had an inadequate response to MTX 1
RINVOQ is indicated for TNFi-IR patients



Upadacitinib 30 mg is not an approved dose.
a Patients on MTX were randomized to receive either RINVOQ 15 mg or upadacitinib 30 mg at Week 14. 3
b Starting at Week 26, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDS, acetaminophen or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 5
c Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD including those previously on UPA 30 mg. 7
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.



*Prior to receiving study drug. In the control arm, patients continued prior MTX dose as blinded study drug.
† Prednisone equivalent
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; CDAI=clinical disease activity index; cMTX=continuous methotrexate; csDMARDs=conventional synthetic disease‑modifying antirheumatic drugs; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C‑reactive protein; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Smolen JS, Pangan AL, Emery P, et al. Supplement - Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT‑MONOTHERAPY): a randomised, placebo‑controlled, double‑blind phase 3 study. Lancet. 2019;393(10188):2303-2311.
3. Smolen JS, Pangan AL, Emery P, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT‑MONOTHERAPY): a randomised, placebo‑controlled, double‑blind phase 3 study. Lancet. 2019;393(10188):2303-2311.
4. Smolen JS, Emery P, Rigby W, et al. Upadacitinib as monotherapy in patients with rheumatoid arthritis: results at 48 weeks from the SELECT‑MONOTHERAPY study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
5. Data on File. ABVRRTI68979.
6. Data on File. ABVRRTI68841.
7. A study comparing upadacitinib (ABT-494) monotherapy to methotrexate (MTX) monotherapy in adults with rheumatoid arthritis (RA) who have an inadequate response to MTX (SELECT-MONOTHERAPY). ClinicalTrials.gov identifier: NCT02706951. https://clinicaltrials.gov/ct2/show/NCT02706951. Updated August 2, 2021. Accessed November 30, 2021.
Adults with moderately to severely active RA who had an inadequate response to csDMARD(s) 1
RINVOQ is indicated for TNFi-IR patients



Upadacitinib 30 mg is not an approved dose.
a Starting at Week 24, patients who did not achieve CDAI ≤10 could have initiated or adjusted corticosteroids, NSAIDS, acetaminophen or ≤2 csDMARDs. Patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 4
b Following a protocol amendment, all patients in the long-term extension received UPA 15 mg QD, including those previously on UPA 30 mg. 7
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.



*Based on prednisone equivalent
ACPA=anti‑citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARD= biologic disease‑modifying antirheumatic drug; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; csDMARD=conventional synthetic disease-modifying anti-rheumatic drug; DAS28-CRP=28 joint disease activity score using C-reactive protein; HAQ-DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C-reactive protein; LDA=low disease activity; MTX=methotrexate; NSAIDs=nonsteroidal anti‑inflammatory drugs; PBO=placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once daily; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale.
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Burmester GR, Kremer JM, Van den Bosch F, et al. Supplement - Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‑modifying anti‑rheumatic drugs (SELECT‑NEXT): a randomised, double‑blind, placebo‑controlled phase 3 trial. Lancet . 2018;391(10139):2503‑2512.
3. Burmester GR, Van den Bosch F, Bessette L, et al. Long‑term safety and efficacy of upadacitinib in patients with rheumatoid arthritis and an inadequate response to csDMARDs: results at 60 weeks from the SELECT‑NEXT study. Poster presented at: The European Congress of Rheumatology, 12‑15 June 2019, Madrid, Spain.
4. Data on File. ABVRRTI68981.
5. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease‑modifying anti‑rheumatic drugs (SELECT‑NEXT): a randomised, double‑blind, placebo‑controlled phase 3 trial. Lancet. 2018;391(10139):2503‑2512.
6. Data on File. ABVRRTI68843.
7. Data On File. ABVRRTI73233.
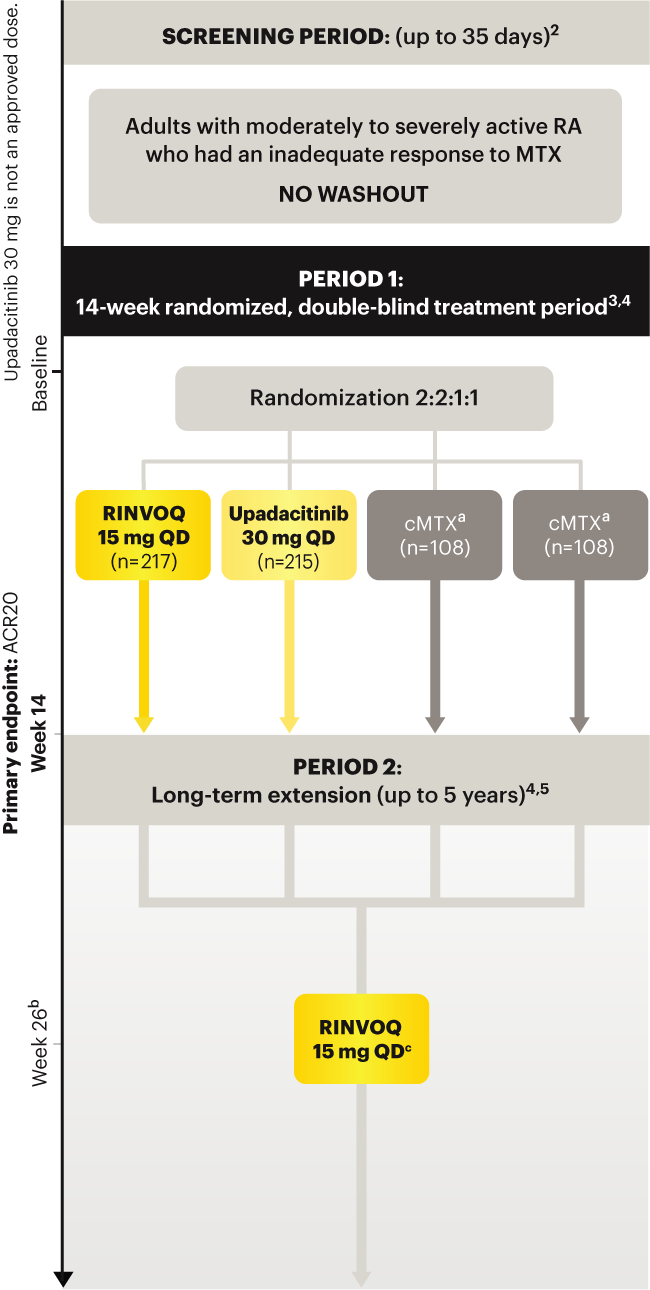
Adults with moderately to severely active RA who had an inadequate response to MTX 1
RINVOQ is indicated for TNFi-IR patients



a X-ray imaging was performed at these time points; Week 14 for non-responder patients, who were rescued. 2,3
b Rescue criteria: At Weeks 14, 18 and 22 if 10. 2
c Starting at Week 26, initiation or change in background RA medication(s) including corticosteroids, NSAIDs, or acetaminophen was permitted. 4
d Starting at Week 48, patients who failed to show ≥20% improvement in TJC and SJC compared to baseline at 2 consecutive visits were removed from the study. 5
e At Week 48, initiation or change in csDMARDs was allowed, however not all patients received background MTX. 5
f Patients continued treatment with UPA or active comparator in a blinded manner until the last patient completed the Week 48 visit and received open-label treatment thereafter. 8
At Week 12 vs Placebo + MTX:
At Week 26 vs Placebo + MTX:
Prespecified nonranked endpoints were not controlled for multiplicity; therefore, treatment differences could represent chance findings. No conclusions regarding these comparisons can be made.
SELECT-COMPARE was not designed to evaluate the efficacy of active comparator + MTX vs Placebo + MTX. No conclusions regarding this comparison can be made.



*Based on prednisone equivalent
ACPA=anti-citrullinated protein antibody; ACR=American College of Rheumatology; ACR20=improvement of at least 20% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR50=improvement of at least 50% in tender joint count, swollen joint count, and at least 3 other core criteria; ACR70=improvement of at least 70% in tender joint count, swollen joint count, and at least 3 other core criteria; bDMARDs=biologic disease‑modifying antirheumatic drugs; CDAI=clinical disease activity index; CR=clinical remission; CRP=C‑reactive protein; DAS28-CRP=28 joint disease activity score using C-reactive protein; DAS28-ESR=28 joint disease activity score using erythrocyte sedimentation rate; ESR=erythrocyte sedimentation rate; HAQ‑DI=health assessment questionnaire disability index; hsCRP=high-sensitivity C‑reactive protein; LDA=low disease activity; mTSS=modified total Sharp score; MTX=methotrexate; PBO=Placebo; PhGA=physician’s global assessment of disease activity; PtGA=patient’s global assessment of disease activity; QD=once per day; RA=rheumatoid arthritis; RF=rheumatoid factor; SD=standard deviation; SDAI=simplified disease activity index; SF‑36 (PCS)=36-item short form health survey physical component summary; SJC66=swollen joint count of 66 joints; TJC68=tender joint count of 68 joints; VAS=visual analog scale
1. RINVOQ [package insert]. North Chicago, IL: AbbVie Inc.
2. Fleischmann R, Pangan AL, Song I-H, et al. Supplement - Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double‑blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788‑1800.
3. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double‑blind, randomized controlled trial. Arthritis Rheumatol. 2019;71(11):1788‑1800.
4. Data on File. ABVRRTI70534.
5. Data on File. ABVRRTI70955.
6. Data on File. ABVRRTI68439.
7. Upadacitinib meets all primary and ranked secondary endpoints including superiority versus adalimumab in phase 3 study in rheumatoid arthritis. AbbVie Inc. https://news.abbvie.com/news/upadacitinib-meets-all-primary-and-ranked-secondary-endpoints-including-superiority-versus-adalimumab-in-phase-3-study-in-rheumatoid-arthritis.htm. April 9, 2018. August 15, 2019.
8. Fleishmann R, Mysler E, Bessette L, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis results at 3 years from the SELECT-COMPARE study. Poster presented at: the American College of Rheumatology Convergence, 5-9 November 2021. E-Congress.
9. Data On File. ABVRRTI68440.
10. Data on File. ABVRRTI70956.
11. Fleischmann R, Pangan AL, Mysler E, et al. A phase 3, randomized, double‑blind study comparing upadacitinib to placebo and to adalimumab, in patients with active rheumatoid arthritis with inadequate response to methotrexate. Oral presentation at: The ACR annual meeting 2018, 19–24 October 2018, Chicago, USA.
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
By clicking “Yes,” I certify that I am a licensed Healthcare Professional and wish to proceed with registering for this educational event.
Do you wish to leave this site?
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
©2024 AbbVie Inc. North Chicago, IL 60064. If you have any questions about this website that have not been answered, click here. This website and the information contained herein is intended for use by US physicians only and is provided for informational purposes only.
IMPORTANT SAFETY INFORMATION & INDICATIONS FOR HUMIRA® (ADALIMUMAB) 1
Rheumatoid Arthritis: HUMIRA is indicated, alone or in combination with methotrexate or other non-biologic DMARDs, for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active rheumatoid arthritis.
Psoriatic Arthritis: HUMIRA is indicated, alone or in combination with non-biologic DMARDs, for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active psoriatic arthritis.
Ankylosing Spondylitis: HUMIRA is indicated for reducing signs and symptoms in adult patients with active ankylosing spondylitis.
IMPORTANT SAFETY INFORMATION 1
Patients treated with HUMIRA are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue HUMIRA if a patient develops a serious infection or sepsis.
Reported infections include:
Carefully consider the risks and benefits of treatment with HUMIRA prior to initiating therapy in patients: 1. with chronic or recurrent infection, 2. who have been exposed to TB, 3. with a history of opportunistic infection, 4. who resided in or traveled in regions where mycoses are endemic, 5. with underlying conditions that may predispose them to infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with HUMIRA, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including HUMIRA. Postmarketing cases of hepatosplenic T‑cell lymphoma (HSTCL), a rare type of T‑cell lymphoma, have been reported in patients treated with TNF blockers, including HUMIRA. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. Almost all of these patients had received treatment with azathioprine or 6‑mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants.
HEPATITIS B VIRUS REACTIVATION
CONGESTIVE HEART FAILURE
Reference: 1. HUMIRA Injection [package insert]. North Chicago, IL: AbbVie Inc.
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
You are leaving an AbbVie website and connecting to a site that is not under the control of AbbVie. AbbVie is not responsible for the contents of any such site or any further links from such site. AbbVie is providing these links to you only as a convenience and the inclusion of any link does not imply the endorsement of the linked site by AbbVie. You should also be aware that the linked site may be governed by its own set of terms and conditions and privacy policy for which AbbVie has no responsibility.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
By selecting "Yes" below, you certify that you are a Healthcare Professional and that you wish to proceed to the Healthcare Professionals Only section on the AbbVie Medical Information site. Products or treatments described on this site are available in the U.S. but may not be available in all other countries. I am a licensed Healthcare Professional and wish to proceed to the Healthcare Professionals Only AbbVie Medical Information Site.
Conversely, the presence of this link does not imply the linked site's endorsement of rinvoqhcp.com or AbbVie.
Do you wish to leave this site?
On January 14, 2022, the Prescribing Information and Medication Guide for RINVOQ (upadacitinib) was updated to include a new Indication, Recommended Dosage information and added a Contraindication related to Hypersensitivity and a new Warning and Precaution for Hypersensitivity Reactions.
The relevant sections of the Prescribing Information read as follows:
1 INDICATION AND USAGE
Section 1.3 Atopic Dermatitis
RINVOQ is indicated for the treatment of adults and pediatric patients 12 years of age and older with refractory, moderate to severe atopic dermatitis whose disease is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies are inadvisable.
Limitations of Use: RINVOQ is not recommended for use in combination with other JAK inhibitors, biologic immunomodulators, or with other immunosuppressants.
2 DOSAGE AND ADMINISTRATION
2.5 Recommended Dosage in Atopic Dermatitis
Pediatric Patients 12 Years of Age and Older Weighing at Least 40 kg and Adults Less Than 65 Years of Age
Initiate treatment with 15 mg once daily. If an adequate response is not achieved, consider increasing the dosage to 30 mg once daily. Discontinue RINVOQ if an adequate response is not achieved with the 30 mg dose. Use the lowest effective dose needed to maintain response.
Adults 65 Years of Age and Older
The recommended dosage is 15 mg once daily.
2.6 Recommended Dosage in Patients with Renal Impairment or Severe Hepatic Impairment
Renal Impairment
Atopic Dermatitis:
Hepatic Impairment
RINVOQ is not recommended for use in patients with severe hepatic impairment.
4 CONTRAINDICATIONS
RINVOQ is contraindicated in patients with known hypersensitivity to upadacitinib or any of its excipients.
5 WARNINGS AND PRECAUTIONS
Section 5.6 Hypersensitivity Reactions
Serious hypersensitivity reactions such as anaphylaxis and angioedema were reported in patients receiving RINVOQ in clinical trials. If a clinically significant hypersensitivity reaction occurs, discontinue RINVOQ and institute appropriate therapy.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients to discontinue RINVOQ and seek immediate medical attention if they develop any signs and symptoms of allergic reactions.
On December 2, 2021, the Prescribing Information and Medication Guide for RINVOQ® (upadacitinib) was updated as a result of discussions with the U.S. Food and Drug Administration (FDA). This follows a Drug Safety Communication (DSC) issued on September 1, 2021, by the FDA based upon its review of a large, randomized, post-marketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another Janus kinase (JAK) inhibitor to tumor necrosis factor (TNF) blockers.
The indication has been updated to the following:
1 INDICATION AND USAGE
Section 1.1 Rheumatoid Arthritis
RINVOQ (upadacitinib) is indicated for the treatment of adults with moderately to severely active rheumatoid arthritis who have had an inadequate response or intolerance to one or more TNF blockers.
Limitation of Use: Use of RINVOQ in combination with other JAK inhibitors, biologic DMARDs, or with potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
The full Prescribing Information now includes new information about the risks of Mortality and Major Adverse Cardiovascular Events and updated information about the risks of Malignancies and Thrombosis within the Boxed Warning and Warnings and Precautions sections.
The Boxed Warning has been updated to the following:
WARNING: SERIOUS INFECTIONS, MORTALITY, MALIGNANCY, MAJOR ADVERSE CARDIOVASCULAR EVENTS, AND THROMBOSIS
SERIOUS INFECTIONS
Patients treated with RINVOQ are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
If a serious infection develops, interrupt RINVOQ until the infection is controlled.
Reported infections include:
The risks and benefits of treatment with RINVOQ should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with RINVOQ, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy.
In a large, randomized, postmarketing safety study in rheumatoid arthritis (RA) patients 50 years of age and older with at least one cardiovascular risk factor comparing another Janus kinase (JAK) inhibitor to tumor necrosis factor (TNF) blockers, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed with the JAK inhibitor.
MALIGNANCIES
Lymphoma and other malignancies have been observed in patients treated with RINVOQ. In RA patients treated with another JAK inhibitor, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk.
MAJOR ADVERSE CARDIOVASCULAR EVENTS
In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of major adverse cardiovascular events (MACE) (defined as cardiovascular death, myocardial infarction, and stroke) was observed when compared with TNF blockers. Patients who are current or past smokers are at additional increased risk. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Thrombosis, including deep venous thrombosis, pulmonary embolism, and arterial thrombosis have occurred in patients treated with JAK inhibitors used to treat inflammatory conditions. Many of these adverse events were serious and some resulted in death. In RA patients 50 years of age and older with at least one cardiovascular risk factor treated with another JAK inhibitor, a higher rate of thrombosis was observed when compared with TNF blockers. Avoid RINVOQ in patients at risk. Patients with symptoms of thrombosis should discontinue RINVOQ and be promptly evaluated.
The following sub-sections in the Warnings and Precautions have been updated to the following:
5 WARNINGS AND PRECAUTIONS
Section 5.2 Mortality
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of all-cause mortality, including sudden cardiovascular death, was observed in patients treated with the JAK inhibitor compared with TNF blockers.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ.
Section 5.3 Malignancy and Lymphoproliferative Disorders
Malignancies were observed in clinical studies of RINVOQ.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients, a higher rate of malignancies (excluding non-melanoma skin cancer [NMSC]) was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lymphomas was observed in patients treated with the JAK inhibitor compared to those treated with TNF blockers. A higher rate of lung cancers was observed in current or past smokers treated with the JAK inhibitor compared to those treated with TNF blockers. In this study, current or past smokers had an additional increased risk of overall malignancies.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients with a known malignancy (other than a successfully treated NMSC), patients who develop a malignancy when on treatment, and patients who are current or past smokers.
Non-Melanoma Skin Cancer
NMSCs have been reported in patients treated with RINVOQ. Periodic skin examination is recommended for patients who are at increased risk for skin cancer.
Section 5.4 Major Adverse Cardiovascular Events
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, a higher rate of major adverse cardiovascular events (MACE) defined as cardiovascular death, non-fatal myocardial infarction (MI), and non-fatal stroke was observed with the JAK inhibitor compared to those treated with TNF blockers. Patients who are current or past smokers are at additional increased risk.
Consider the benefits and risks for the individual patient prior to initiating or continuing therapy with RINVOQ, particularly in patients who are current or past smokers and patients with other cardiovascular risk factors. Patients should be informed about the symptoms of serious cardiovascular events and the steps to take if they occur. Discontinue RINVOQ in patients that have experienced a myocardial infarction or stroke.
Section 5.5 Thrombosis
Thrombosis, including deep venous thrombosis (DVT), pulmonary embolism (PE), and arterial thrombosis, have occurred in patients treated for inflammatory conditions with JAK inhibitors, including RINVOQ. Many of these adverse events were serious and some resulted in death.
In a large, randomized, postmarketing safety study of another JAK inhibitor in RA patients 50 years of age and older with at least one cardiovascular risk factor, higher rates of overall thrombosis, DVT, and PE were observed compared to those treated with TNF blockers.
If symptoms of thrombosis occur, patients should discontinue RINVOQ and be evaluated promptly and treated appropriately. Avoid RINVOQ in patients that may be at increased risk of thrombosis.
This information was added to the following section:
17 PATIENT COUNSELING INFORMATION
Major Adverse Cardiovascular Events
Inform patients that RINVOQ may increase their risk of major adverse cardiovascular events (MACE) including myocardial infarction, stroke, and cardiovascular death. Instruct all patients, especially current or past smokers or patients with other cardiovascular risk factors, to be alert for the development of signs and symptoms of cardiovascular events.
The following information on important labeling revisions does not include all changes; please refer to the RINVOQ full Prescribing Information.
Indications and Important Safety Information for RINVOQ (upadacitinib) 1